Abstract
Neisseria gonorrhoeae can invade into cervical epithelial cells to overcome this host defense barrier. We developed a β-lactamase reporter system that allowed us to quantify at the single cell level if a host cell internalized a viable or nonviable microorganism. We autodisplayed β-lactamase on the surface of FA1090 [FA1090Φ(bla-iga′)] and demonstrated by confocal fluorescence microscopy and flow cytometry that FA1090Φ(bla-iga′) cleaved the β-lactamase substrate CCF2-AM loaded into host cells only when gonococci were internalized by these host cells. While FA1090Φ(bla-iga′) adhered to almost all ME180 cells, viable N. gonorrhoeae were internalized by only a subset of cells during infection. Nonviable gonococci adhered to, but were not internalized by ME180 cells and failed to recruit F-actin to sites of adherent bacteria. Overall, we show that epithelial cell invasion is a dynamic process that requires viable N. gonorrhoeae. We demonstrate the advantages of the β-lactamase reporter system over the gentamicin protection assay in quantifying bacterial invasion. The reporter system that we have developed can be adapted to studying the internalization of any bacterial species into any host cell.
Keywords: Autodisplay, Beta-lactamase, CCF2-AM, F-actin, Invasion/Internalization, Neisseria gonorrhoeae
1. Introduction
Neisseria gonorrhoeae (gonococci, or GC), the causative agent of the sexually transmitted disease gonorrhea, colonize mucosal epithelia and endothelia and trigger an intense inflammatory response characterized by neutrophil influx. GC may cross the mucosal barrier by triggering their own internalization into cervical endothelial cells (for review, see [1]). Internalization requires both bacterial and host cell factors [2]. Pili, in conjunction with one or more gonococcal invasins (opacity proteins [Opa], lipooligosaccharide [LOS], and/or porin) or iC3b surface deposition, can induce changes in host cell signaling to drive host filamentous actin (F-actin) polymerization beneath adherent GC, triggering microvilli elongations that promote internalization [3–8]. Scanning electron micrographs revealed that nonviable GC fail to induce microvilli elongation in Hec1B cervical epithelial cells, and visual internalization of dead GC by these cells was not seen [3]. The F-actin rearrangements normally observed in epithelial cells during the internalization of viable GC into epithelial cells are not seen during the interaction of dead GC with various epithelial cell lines [9].
Current methods to measure GC internalization into host epithelial cells rely on the quantification of intracellular bacteria through adaptations of the gentamicin protection assay [10]. Gonococci are deemed to be internalized if they survive after gentamicin is added to infected host cells [10, 11]. Quantifying GC internalization using the gentamicin protection assay possesses inherent limitations: it does not measure the number of host cells that contain internalized GC; it does not measure the frequency by which GC are internalized by host cells; it cannot determine if nonviable GC are capable of entering into host cells; and it underestimates the number of intracellular GC if the internalized bacteria aggregate within host cells or overestimates the number of internalized GC if not all extracellular GC are killed during gentamicin treatment.
In the present study, we established a gonococcal reporter strain that expresses a β-lactamase ((Bla)-IgA protease β-domain (IgAβ) fusion protein) in a FA1090 background using the strategy of autotransporter-mediated surface display (autodisplay). This strategy allows heterologous passenger domains, such as Bla, to be expressed on the outer surface of bacteria when fused with an autotransporter protein. Using the FA1090Φ(bla-iga′) strain, we demonstrate that only a subpopulation of human cervical epithelial cells (ME180) internalize viable GC and show that gonococcal viability is required for GC internalization into host cells.
2. Materials and Methods
2.1 Bacterial strains, cell lines, plasmids, and reagents
GC strain FA1090 was obtained from Dr. W. M. Shafer (Emory University, Atlanta, GA) and maintained on gonococcal media base (GCK) (Becton Dickinson, Sparks, MD) containing 5 g/L agar and 1% Kellogg’s supplement [12]). Penicillin G (Pen) was added to GCK agar plates as indicated. GC were killed with gentamicin sulfate (BioWhittaker/Cambrex Biosciences, Walkersville, MD) as described previously [13]. Piliated (Pil+), Opa-expressing (Opa+) GC strains were used in all experiments and colonies exhibiting these phenotypes were selected based on the light refraction properties of GC colonies on agar plates using a dissecting light microscope. Escherichia coli strain DH5α mcr− was obtained from New England Biolabs (Beverly, MA). The chimeric Bla plasmid pFT180 [14] and pK18UP [15] have been described previously. ME180 cells, a human cervical epidermal carcinoma cell line (HTB-33; American Type Culture Collection, Manassas, VA), were maintained in RPMI 1640 (Gibco/Invitrogen, Grand Island, NY) supplemented with 5% fetal bovine serum (FBS) (HyClone, Logan, UT), 1% Pen (1000 U/ml stock)-streptomycin (10 mg/ml stock) solution (Gibco/Invitrogen), and 10 μM Hepes (Mediatech, Herndon, VA). Chemicals used in this study were reagent grade or better and were obtained from Sigma Aldrich (St. Louis, MO) unless otherwise specified.
2.2 Establishing the reporter strain, FA1090Φ(bla-iga′)
The Bla-IgAβ fusion protein was constructed using a gene replacement strategy (Fig. 1A) and was expressed in FA1090 to yield strain FA1090Φ(bla-iga′). FA1090 chromosomal DNA was purified with a kit from Promega (Madison, WI). Transformants were selected on GCK agar containing 10 ng/ml Pen (GCK-Pen). (All primers used in this study are described in Table 1.)
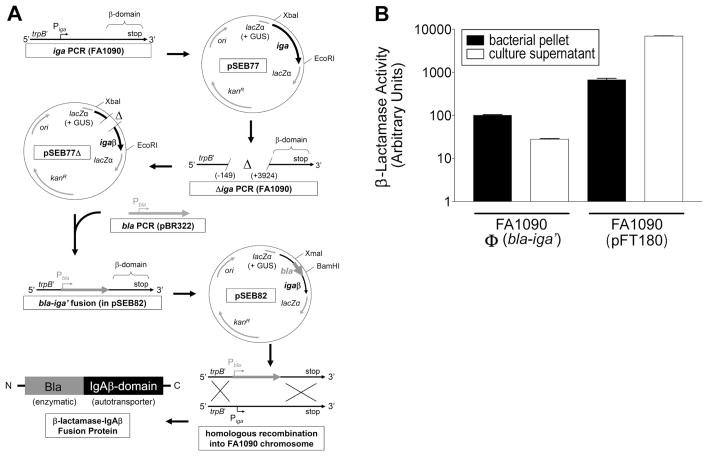
Figure 1.
GC strain FA1090Φ(bla-iga′) expresses the outer membrane anchored Bla-IgAβ fusion protein. (A) The cloning strategy to construct Bla-IgAβ is shown. The IgA protease gene (iga), was amplified by the PCR from FA1090 chromosomal DNA using primers iga-beta F2 and iga-beta R2. The iga PCR product was digested with EcoRI and XbaI and directionally cloned into pK18UP forming plasmid pSEB77 (GUS = gonococcal uptake sequence). The −149 to +3924 bp region of iga was deleted from pSEB77 utilizing deletion PCR with primers iga-beta del F and iga-beta del R. The deletion PCR product was digested with the BamHI and religated onto itself (pSEB77Δ). The β-lactamase (bla) gene (excluding the stop codon sequence) was amplified via the PCR from pBR322 using primers bla F and bla R, digested with BamH1 and XmaI, and directionally cloned into the deletion site of pSEB77Δ to generate the bla-iga′ gene fusion (pSEB82). Strain FA1090 was transformed with pSEB82. Transformants where the bla-iga′ construct replaced the iga gene in the FA1090 chromosome via homologous recombination [FA1090Φ(bla-iga′)] were selected on GCK Pen. (B) Pellets (black bars) or culture supernatants (white bars) of GC cultures were incubated with 50 μg/ml nitrocefin at room temperature for 30 min. Reactions were immediately centrifuged and the OD520 nm of the supernatants was measured. The Bla activity for the FA1090Φ(bla-iga′) pellet in a selected trial was defined as 100 aU and used as the standard to normalize the Bla activity of all other samples. The data are representative of three independent experiments performed in triplicate.
Table 1.
List of primers used in this study
| Primers | DNA Sequence (5′ to 3′)a |
|---|---|
| Bla F | TCCCCCCGGGCCGCATTAAAGCTTATCGATGb |
| Bla R | CGGGATCCCCAATGCTTAATCAGTGAGGc |
| igaβ F2 | GCTCTAGACCCGAGATACTGACCAAATGd |
| igaβ R2 | CGGAATTCCAAAGGCATTGAGCTGTAGCe |
| igaβ del F | CGGGATCCCCCGGGCTGTTATTCAAGCGAAGATGGGAAGCf |
| igaβ del R | CGGGATCCGTACAGCAAAACAATGTGGAAATTGCg |
The underlined sequence represents the restriction enzyme site
Amplifies bla starting 250 bp upstream of bla start codon, XmaI site
Amplifies bla starting at the 3′ end of bla excluding the stop codon, BamHI site
Amplifies iga starting 1095 bp upstream of iga start codon, XbaI site
Amplifies iga starting 94 bp downstream of iga stop codon, EcoRI site
Amplifies sequence starting 149 bp upstream of the iga promoter and continues and continues upstream of iga, BamHI site
Amplifies the β-domain of iga and downstream sequence, BamHI site
2.3 Nitrocefin Hydrolysis Assay
Bla activity was determined using a modified version of a spectrophotometric assay established by O’Callaghan, et al. [16]. Briefly, GC were grown overnight in broth at 37°C, resuspended to a turbidity of 100 Klett units (green filter) as measured by a Klett-Summerson colorimeter, and collected by centrifugation. Pellets were resuspended in 0.1 M phosphate buffer, 1 mM EDTA, pH 7.0 and supernatants were diluted 10-fold in the same buffer solution. A 500 μg/ml stock solution of nitrocefin (Calbiochem, LaJolla, CA) was prepared according to the manufacturer’s instructions. Dilutions of bacteria, culture supernatants, or buffer controls were incubated with nitrocefin (fc 50 μg/ml) (RT, 30 min). The absorbance at 520 nm was measured. The Bla activity of 100 arbitrary units (aU) is defined as the amount of nitrocefin hydrolyzed in 30 min by 1 × 109 FA1090Φ(bla-iga′) in a 1 ml reaction. Enzymatic activity in all other samples was normalized to this reading.
2.4 Gentamicin Protection Assay
ME180 cervical epithelial cells were seeded in 24-well tissue culture plates at 1 × 105 cells/well and incubated overnight at 37°C in 5% CO2. Prior to use, cells were viewed under a light microscope to ensure they had reached ~80% confluency in the well. Bacteria were also grown overnight on GCK agar, resuspended to a turbidity of 100 Klett units, added to ME180 cells at an MOI of 10, and incubated in internalization media (IM) (RPMI, 5% FBS, 0.5% Kellogg’s supplement, and 10 mM Hepes) for 6 h. Cells were washed four times with IM. To quantify the total number of host cell associated GC, including adherent and internalized GC, cells were treated with 1% saponin (15 min) and aliquots plated on GCK agar. To quantify the number of internalized GC, gentamicin (200 μg/ml) was added 2 h prior to the preparation of ME180 cell lysates. The number of CFU arising on GCK agar was determined after 36–48 h incubation.
2.5 Immunofluorescence Microscopy Analysis
ME180 cells were seeded on coverslips in 24-well plates overnight, incubated with GC for various lengths of time, washed three times with IM and once with phosphate-buffered saline (PBS), fixed (20 min) with 4% paraformaldehyde (Electron Microscopy Sciences, Ft. Washington, PA) and treated with blocking solution (10% FBS and 10 μM glycine in PBS, 5 min). Extracellular, adherent GC were stained with an Oregon green conjugated goat anti-GC antibody (Ab) (4°C, 60 min). Cells were washed three times with PBS and treated with a permeabilization solution (0.1% Triton X-100, 10% FBS, 10 mM glycine in PBS) for 5 min at RT. F-actin was stained with Alexa Fluor 546-labeled phalloidin (4 U/ml) (Molecular Probes/Invitrogen) in the permeabilization solution (4°C, 60 min). Cells were washed with PBS, fixed with 2% paraformaldehyde, mounted on glass slides and analyzed using a Zeiss LSM 510 laser scanning confocal microscope.
2.6 Cellular Distribution of CCF2-AM
ME180 cells were seeded in 24-well plates as described above and washed with RPMI 1640 containing 10 μM Hepes. To determine if CCF2-AM can enter nuclear compartments, ME180 cells were loaded with 1 μM CCF2-AM (Invitrogen/PanVera, Carlsbad, CA) (90 min, RT) and Hoechst 33342 (100 ng/ml) (Molecular Probes/Invitrogen) was added for the final 60 min. To analyze whether CCF2-AM can enter into endocytic compartments, cells were stained with 50 nM LysoTracker Red DND-99 (Molecular Probes) (30 min, 37°C), transferred to RT, and then co-loaded with 1 μM CCF2-AM for an additional 90 min. Following both staining procedures, cells were washed once with PBS and mounted on glass slides with gel mount. Cells were analyzed using a Zeiss Axioplan 2 fluorescence microscope.
2.7 Fluorescence-based β-lactamase reporter assay
ME180 cells were seeded in 6-well plates without coverslips overnight. FA1090Φ(bla-iga′) was grown on GCK-Pen overnight, added to the cells at the indicated MOI, and incubated for 6 h (unless otherwise stated). GC culture supernatants were prepared as above and filtered through 0.2 μm syringe filter. After washing, cells were incubated with 0.25 μM CCF2-AM for 45–60 min. For flow cytometry, cells were washed with PBS, incubated in 0.05% trypsin/0.53 mM EDTA (15 min, 37°C). Cells were resuspended in PBS and analyzed using a CyAn ADP flow cytometer (Dako Cytomation, Fort Collins, CO) Live, single cells were gated, and the fluorescence intensities of cleaved and uncleaved CCF2-AM of individual cells were detected using 405 nm and 488 nm excitation lasers, respectively. Data were analyzed using the Summit 4.1 software (Dako cyomation).
Results
3.1 Cell association of GC-expressed β-lactamase
The β-lactamase-IgA protease β-domain (Bla-IgAβ) fusion protein expressed in FA1090Φ(bla-iga′) was constructed using the cloning strategy outlined in Fig. 1A. We replaced the protease domain of IgA protease with Bla and utilized theβ-domain to localize Bla on the extracellular face of the GC outer membrane. To demonstrate that Bla is cell-associated in FA1090Φ(bla-iga′), we compared FA1090Φ(bla-iga′) nitrocefin hydrolysis activity to FA1090(pFT180), which expresses high levels of soluble Bla (Fig 1B). Most (~80%) of the enzymatic activity from FA1090Φ(bla-iga′) was localized in the bacterial pellet (100 arbitrary units [aU] compared to <30 aU of Bla activity in the culture supernatant), whereas in FA1090(pFT180), most (~90%) of the Bla activity was detected in the culture supernatant (~7000 aU compared to ~700 aU in the bacterial pellet) (Fig. 1B). These data indicate that Bla is cell-associated in FA1090Φ(bla-iga′) and secreted by FA1090(pFT180).
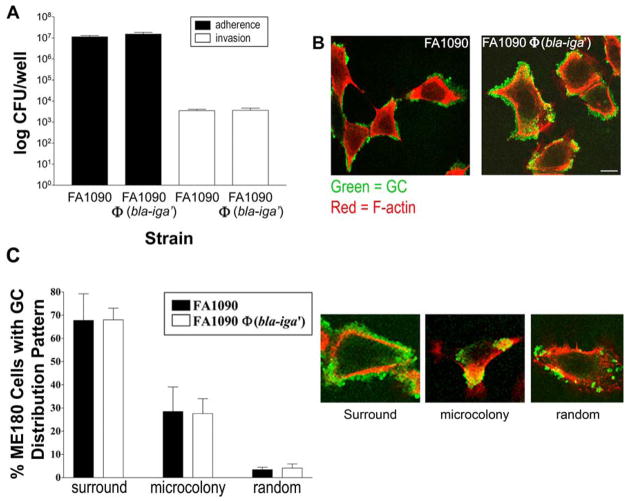
To determine if Bla autodisplay alters FA1090’s ability to adhere to and/or be internalized by cervical epithelial cells, we performed a gentamicin protection assay. The data indicate that a similar number of FA1090 and FA1090Φ(bla-iga′) adhered to and were internalized by ME180 cells (Fig. 2A). (Control experiments revealed that gentamicin exhibits equivalent killing effects on extracellular FA1090 and FA1090Φ(bla-iga′) leaving a minimal level of background extracellular GC prior to measuring GC internalization [data not shown].) Furthermore, laser scanning confocal microscopy (LSCM) analysis revealed the majority of FA1090 and FA1090Φ(bla-iga′) bacteria were observed surrounding ME180 cells (Fig. 2B), and the overall pattern of distribution of bacteria was similar between the two strains (Fig. 2C). These data indicate that Bla expression and deletion of the IgA protease domain by FA1090Φ(bla-iga′) do not interfere with its host cell interactions.
Figure 2.
FA1090Φ(bla-iga′) and FA1090 interact with ME180 cells in a similar manner. (A) ME180 cells were incubated with GC at a MOI 10 for 6 h at 37°C, 5% CO2 and then incubated for 2 h in media alone to quantify adherent GC (black bars) or media containing gentamicin (200 μg/ml) to quantify internalized GC (white bars). Cells were lysed and lysates were plated by serial dilution for viable GC plate count. Shown are representative data of three independent experiments performed in triplicate (±SD). (B) ME180 cells were incubated with GC for 6 h.. Extracellular GC were stained with an Oregon green-conjugated anti-GC Ab and F-actin was stained with Alexa Fluor 546-labeled phalloidin prior to analysis via LSCM. Bar, 10 μm. (C) FA1090 (black bars) and FA1090Φ(bla-iga′) (white bars) from (B) were scored on their cellular distribution patterns on a total of approximately 300 randomly selected ME180 cells. GC distributions were scored as layers of GC surrounding the cell (surround), microcolon(ies) on the cell (microcolony), or randomly distributed individual GC or small clusters (random). The LSCM images provide a visual representation of each distribution. Data represent the average (± SD) percentage of ME180 cells that display each distribution from three independent experiments. Pil+Opa+ GC were used in all trials.
3.2 Analysis of ME180 cells that internalized GC
We utilized FA1090Φ(bla-iga′) to quantify the number of ME180 cells that internalized GC. When Bla+ GC are present inside CCF2-AM-loaded ME180 cells, they will cleave the intracellular CCF2-AM (green fluoresence) to yield coumarin (blue fluorescence), whereas in cells without internalized Bla+ GC, CCF2-AM will remain uncleaved (Fig 3A). To examine the intracellular distribution of CCF2-AM, we analyzed uninfected CCF2-AM-loaded ME180 cells by fluorescence microscopy. Since CCF2-AM colocalized with Hoechst 33342, it is clear that this dye has access to the nuclear compartments of ME180 cells (Fig. 3B). We allowed ME180 cells to take up LysoTracker in the presence of CCF2-AM, and found that the CCF2-AM and LysoTracker dyes colocalized with each other (Fig. 3C), indicating that CCF2-AM can be taken up into endocytic compartments. These results demonstrated that in addition to the cytoplasm, CCF2-AM can enter most of the intracellular membrane compartments.
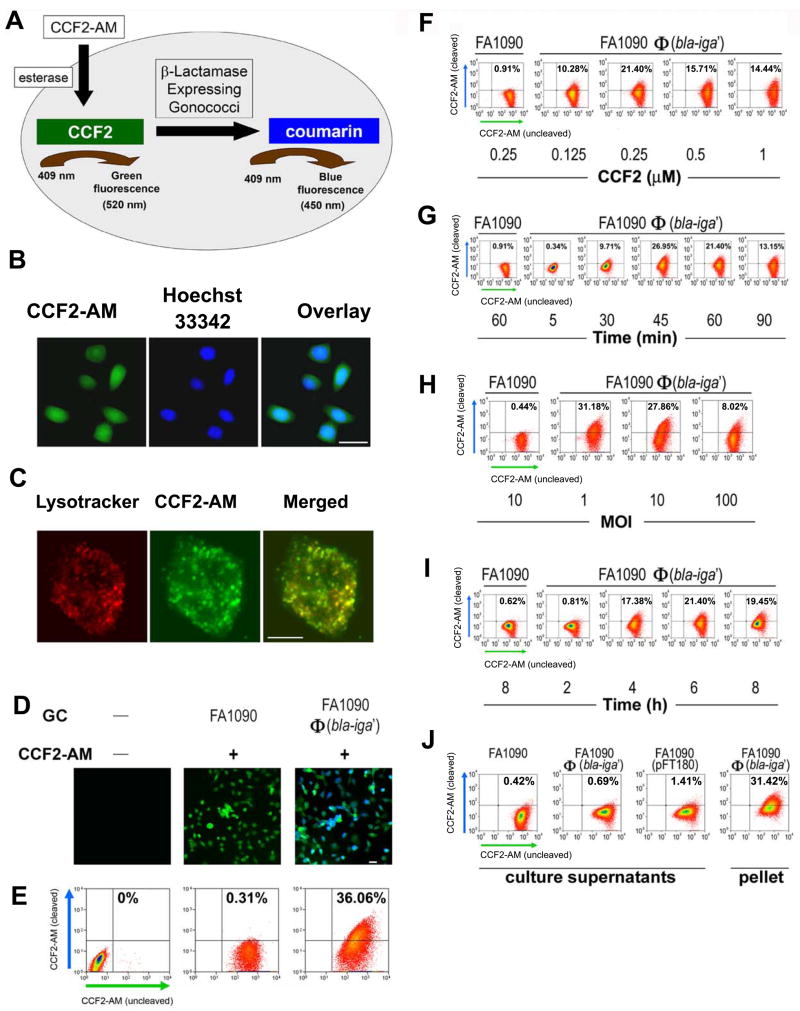
Figure 3.
Analyses of FA1090Φ(bla-iga′) internalization by ME180 cells utilizing the Bla reporter assay. (A) An overview of the internalization detection process in the Bla reporter assay. (B) ME180 cells were incubated simultaneously at room temperature with 1 μM CCF2-AM (green) for 90 min and Hoechst 33342 (blue) (100 ng/ml) for the final 60 min. Bar, 20 μm. (C) ME180 cells were incubated with 50 nM LysoTracker Red DND-99 for 30 min at 37°C and cells were shifted to room temperature where they were loaded with 1 μM CCF2-AM for an additional 90 min. Bar, 5 μm. (D) ME180 cells were incubated with or without GC for 6 h at 37°C, 5% CO2 and afterwards loaded with or without 1 μM CCF2-AM for 90 min. Bar, 20 μm. In (E–J), 1 × 106 ME180 cells/well were infected with FA1090Φ(bla-iga′) at an MOI 10 or culture supernatants derived from cultures containing 1 × 108 GC/ml (J) at 37°C, 5% CO2 for 6 h (unless otherwise indicated). For flow cytometry analysis (E–J), ME180 cells were then incubated with 0.25 μM CCF2-AM for 45–60 min. (F) Infected ME180 cells were loaded with different concentrations of CCF2-AM for 45–60 min. (G) Infected ME180 cells were loaded with CCF2-AM for different lengths of time. (H) ME180 cells were incubated with GC at different MOIs before being loaded with CCF2-AM. (I) ME180 cells were incubated with GC for different lengths of time before being stained with CCF2-AM. (J) ME180 cells were incubated with filtered supernatants (with varied levels of Bla activity) derived from overnight broth cultures of wild-type FA1090 (<1 aU), FA1090Φ(bla-iga′) (<3 aU), or FA1090(pFT180) (~700 aU) and then loaded with CCF2-AM. ME180 cells were incubated with FA1090Φ(bla-iga′) at an MOI 10 (10 aU) as a positive control for CCF2-AM cleavage in part H. In (E–J), ME180 cells incubated with FA1090 or FA1090 culture supernatant served as flow cytometry gating controls and all flow cytometry dot plots shown are representative data of two to five independent experiments. All GC used in these experiments were Pil+Opa+. Image quality was optimized using the AxioVision 4.6 software release from Carl Zeiss North America.
To identify individual host cells that internalized GC, ME180 cells were infected with FA1090Φ(bla-iga′) and incubated with CCF2-AM. Fluorescence microscopy showed that only ME180 cells inoculated with FA1090Φ(bla-iga′) exhibited detectable levels of cleaved CCF2-AM (blue fluorescence) (Fig. 3D). Using flow cytometry, we determined the percentage of ME180 cells that were positive for cleaved CCF2-AM and found that less than 40% of FA1090Φ(bla-iga′)-infected ME180 cells showed higher levels of cleaved CCF2-AM than negative controls (Fig. 3E). These data indicate that only a subpopulation of ME180 cells internalize FA1090Φ(bla-iga′).
We determined the optimal conditions needed to quantify GC internalization using the Bla reporter assay. We found that a minimum of 0.25 μM CCF2-AM and 45–60 min dye loading were needed for optimal detection of ME180 cells containing internalized GC (Fig. 3F and 3G). Increasing the MOI from 1 to 10 did not alter the internalization efficiency, but at an MOI of 100 GC/cell the subpopulation of host cells that internalized GC decreased (Fig. 3H). The results of a time course analysis for GC internalization were consistent with the internalization kinetics reported for the gentamicin protection assay and microscopic visualization assays utilized to quantify GC internalization (Fig. 3I) [10, 17–20]. The number of ME180 cells that internalized FA1090Φ(bla-iga′) reached its maximal level by 4–6 h and did not increase with continued incubation (Fig. 3I). To demonstrate that CCF2 cleavage was the result of FA1090Φ(bla-iga′) internalization and not pinocytosis of secreted Bla or GC membrane blebbing, we incubated ME180 cells with FA1090(pFT180) culture supernatants (~7000 aU). Minimal CCF2-AM cleavage was detected in ME180 cells treated with FA1090(pFT180) supernatants (Fig. 3J). These results demonstrate that cleavage of intracellular CCF2-AM is the direct result of FA1090Φ(bla-iga′) internalization and not due to the uptake of Bla secreted by GC.
3.3 Analysis of the internalization of nonviable GC and their ability to recruit actin
We determined if GC viability is required for GC internalization by ME180 cells. FA1090Φ(bla-iga′) was killed with gentamicin to generate nonviable bacteria with fixed, unchangeable surface structure profiles that cannot vary during their interactions with host cells [13]. Gentamicin-killed FA1090Φ(bla-iga′) maintained Bla activity similar to viable FA1090Φ(bla-iga′) (Fig. 4A). We tested if the physical interaction of GC surface molecules with ME180 cells in the absence of bacterial viability is sufficient to induce GC uptake. At all MOIs tested (1–1000), no significant amount of blue fluorescent (coumarin-positive) ME180 cells were detected when incubated with gentamicin-killed FA1090Φ(bla-iga′) (Fig. 4B), indicating that GC viability is required for GC internalization by cervical epithelial cells.
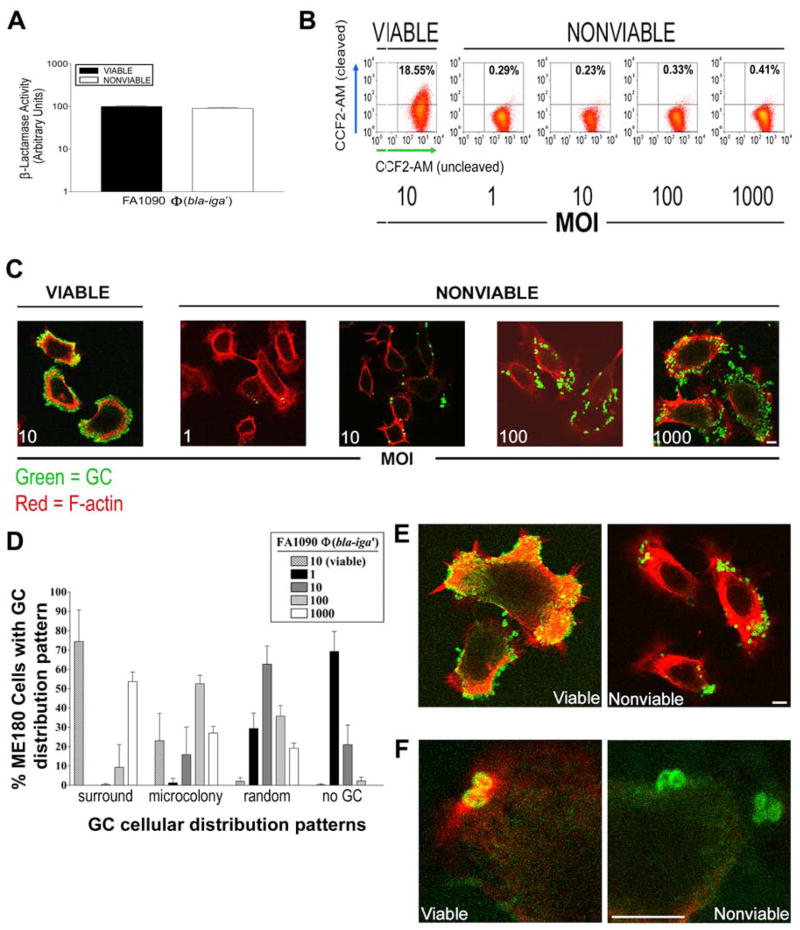
Figure 4.
GC viability is required for host cell F-actin recruitment and subsequent GC internalization by ME180 cells. (A) ME180 cells were incubated with nonviable FA1090Φ(bla-iga′) at different MOI for 6 h and loaded with 0.25 μM CCF2-AM for 45–60 min. ME180 cells incubated with viable FA1090Φ(bla-iga′) at an MOI 10 served as a positive control for internalization/CCF2-AM cleavage. Shown are representative data of five independent experiments. (B) LSCM analyses of the cellular distribution pattern of nonviable FA1090Φ(bla-iga′) at each MOI from part (A). Extracellular GC and F-actin were stained as described in Fig. 2B. (C) Quantification of the GC cellular distribution patterns on approximately 200 randomly selected ME180 cells from the LSCM images in (B) were scored as described in Fig. 2C. Data represents the average (± SD) percentage of ME180 cells that display each distribution pattern from three independent experiments. (D) Shown are higher magnification images of ME180 cells incubated with viable FA1090Φ(bla-iga′) at an MOI 10 (left panel) or nonviable FA1090Φ(bla-iga′) at an MOI 100 (right panel) as in (B). (E) Shown are highly magnified regions of ME180 cells associated with individual viable FA1090Φ(bla-iga′) (left panel) or nonviable FA1090Φ(bla-iga′) (right panel) stained as described in Fig. 2B. Pil+Opa+ FA1090Φ(bla-iga′) were used in all trials. Bars, 5 μm.
To determine why nonviable GC failed to be internalized by cervical epithelial cells, we used fluorescent microscopy to examine the interaction of viable or gentamicin-killed FA1090Φ(bla-iga′) with ME180 cells and GC-induced actin cytoskeleton redistribution. Gentamicin-killed GC attached to ME180 cells, indicating that nonviable GC can adhere to host cells (Fig. 4C and 4D). However, the distribution of killed bacteria seemed to differ from that obtained with viable GC, in that viable cells appeared to form tight associations with the cells, while dead cells seemed to be more loosely associated with the cells. These data suggest that the surface structure profile of viable GC differs from the profile of gentamicin-killed GC and that the surface of viable GC is more suitable for intimate attachment to host cells. The actin cytoskeleton of ME180 cells interacting with gentamicin-killed GC was evenly distributed beneath the plasma membrane (Fig. 4E). This differed from what was seen with cells interacting with live bacteria where F-actin was recruited beneath sites of adherent GC (even under the adherence sites of individual live GC (Fig. 4F)). These data suggest that nonviable GC fail to recruit F-actin and, consequently, are not internalized by host cells.
4. Discussion
GC utilize multiple surface molecules for initiating cross-talk with host cells to activate signaling pathways and alter gene expression in both participants. If physical adhesion to host cells was the only prerequisite for invasion, then gentamicin-killed GC should be internalized by host cells. Our data show that gentamicin-killed piliated FA1090Φ(bla-iga′) can adhere to, but are not internalized by ME180 cells, demonstrating that the physical adherence of bacteria to host cells does not account for the inability of nonviable GC to enter into these cells. However, even though both viable and nonviable GC can attach to host cells, our data also show that the cellular association pattern and internalization fate of adherent viable GC differs from nonviable GC. These data suggest that viable GC can alter their surface adhesin/invasin profile during their interactions with host cells. Gentamicin treatment prevents GC from changing their surface in response to host cells, and modifies the nature of their adherence to host cells. This, in turn, could inhibit the internalization of nonviable GC by preventing them from engaging the proper host cell receptors in the proper sequence to trigger signaling pathways that elicit internalization. Furthermore, our data demonstrated that viable GC were only internalized by a subpopulation of human cervical epithelial cells, even though GC were observed to be adherent to all cells in the population. These findings indicate that the molecular cross-talk between GC and host cells that leads to internalization requires GC viability in addition to the appropriate surface ligand-receptor interactions that trigger host cell signaling pathways required for GC invasion.
The loss of other pilus functions could explain why nonviable GC fail to recruit F-actin after adhering to epithelial cells. When viable GC contact host cells, GC retract pili powered by the ATPase motor protein, PilT [21], triggering the phosphatidyl inositol-3-kinase (PI3K)/Akt signaling pathway in epithelial cells. Blocking this retraction-induced signaling inhibits invasion [22]. Since gentamicin-treated GC are metabolically inactive, they cannot generate ATP to drive pilus retraction. Without pilus retraction, nonviable GC would not trigger retraction-induced signaling in host cells or may not form intimate adhesions with host cells to establish subsequent receptor-ligand interactions essential for GC entry.
The loss of pilus retraction alone cannot explain the inability of nonviable GC to enter into host cells. A previous study showed that blocking protein synthesis in FA1090 with low doses of chloramphenicol prior to infection prevented microvilli elongation in Hec1B cells and inhibited GC internalization, suggesting that new invasion-promoting factors are synthesized during host cell interactions [3]. The de novo synthesis of these factors could be a key event that allows viable GC to trigger host cell signaling pathways that lead to internalization. The GC protein synthesis study above was limited to the small number of cells that could be examined by electron microscopy. Since changes in GC gene regulation occur when GC interact with epithelial cells [23–25], it is difficult to identify the specific changes in GC gene expression that are required for internalization. The Bla reporter system that we have developed has the potential to overcome this challenge. Using the Bla reporter system, we can identify and enrich for host cell-associated GC and compare the gene expression profile of wild type and mutant GC that form intimate attachments to host cells, but fail to gain entry into these cells.
The use of nonviable GC also represents an effective approach for understanding several aspects of GC internalization. In our studies, nonviable GC provide a clean negative control for invasion since no ME180 cells in an infected population internalized gentamicin-killed GC. The lack of host cell entry by nonviable GC demonstrates that invasion is an active process requiring dynamic interplay between the host and pathogen. While it is not know if nonviable GC contribute to GC disease, nonviable GC have been shown to elicit a proinflammatory cytokine response even at low inoculum levels [13]. In women, GC can spread from their initial site of infection to secondary tissues [5, 26]. GC killed during infection would be exposed to mucosal immune cells at these secondary sites since nonviable GC would not be internalized by epithelial cells. Therefore, as GC are progressively killed by host defenses, the nonviable GC may generate immune responses and contribute to tissue damage.
The gentamicin protection assay represents a well accepted assay for quantifying bacterial internalization into host cells [10]. This assay has helped reveal many aspects of GC internalization, yet it is restricted in measuring the cellular details of the internalization process. The assay does not provide direct information about the infected host cells, including the percentage of host cells that internalize GC. Pils, et al. previously developed a method where they fluorescently pre-labeled GC and used flow cytometry to detect bacteria within host cells [27]. This approach is limited to investigating internalization over a short time course (1–2 h) due to the brief lifetime of the fluorescence probe in live GC. In this study, we used flow cytometry and the Bla reporter system to distinguish host cells that internalized GC from cells that did not internalize GC at the single cell level. The Bla reporter system allowed us to quantify internalization events independent of antibiotic resistance, bacterial viability, and the internalization time course. Our data revealed that, even though nearly 100% of ME180 cells were colonized by viable adherent piliated FA1090Φ(bla-iga′) by 4–6 h, the percentage of cells in the population that contained internalized GC never exceeded 40%. Our data also suggest that GC reach a maximum internalization threshold at lower MOI and these lower MOI probably reflect more physiologically relevant infection conditions. At higher MOI (i.e. 100), GC form large microcolonies that could impede their internalization into host cells. Conversely, greater initial GC internalization may occur at the higher MOI, but these highly invaded host cells could be selectively killed by heavy loads of bacteria. Together, these data indicate that GC only trigger invasion into a subset of cervical epithelial. These findings also imply that not all GC-host cell interactions produce the proper sequence of cross-talk responses to yield internalization. The intracellular site(s) for GC could depend on the invasion mechanism utilized by GC and the type of host cell that internalizes the bacteria. Previous studies have shown that GC can be localized in endocytic compartments [4, 8, 28], yet other studies revealed GC gain access to the cytosol in some cell types, including ME180 cells [29]. Because CCF2-AM taken up by host cells can be found in all cellular compartments, the use of this reporter did not allow us to determine where GC were localized in ME180 cells after internalization.
While the Bla reporter assay and the gentamicin protection assay examine GC internalization in different ways, the data from these two assays show similar GC internalization kinetics. Data from the Bla reporter assay and the gentamicin protection assay show that GC internalization takes 4–6 h [10, 17–19]. Utilizing the Bla reporter assay, which examines GC internalization from the host cell perspective, and the gentamicin protection assay, which examines GC internalization from the bacterial angle, provides a more complete understanding of the GC invasion process. To our knowledge, these data present the first study where Bla has been used as an intracellular reporter to analyze internalization of whole bacteria and the first study where the potential internalization of nonviable bacteria has been directly quantified. The Bla reporter system provides an exciting opportunity to further analyze cellular events that are induced by GC and will allow us to dissect the role of individual virulence determinants in the invasion process at the single cell level. This technology should be expandable to quantify internalization into any cell type, by any intracellular bacteria, and on any time scale, providing a new approach to resolving the cellular and molecular details of host cell invasion.
Acknowledgments
This work was supported by grants AI048703 and AI068888 from the National Institutes of Health to DCS and WS. The authors thank those individuals who provided key materials and expertise: Ruth Redman, Jennifer Malloy, Elise Shumsky, and Dan Stevens from Carl Zeiss North America for assistance with immunofluorescence microscopy, Amy Beaven and Dr. Robert Brown from the University of Maryland for help with laser scanning confocal microscopy, and Dr. Susan Pierce from the National Institute of Allergy and Infectious Diseases for providing the flow cytometer. The authors thank Dr. J. MacLeod Griffiss, Dr. Julia Patrone, and Karen Swanson for helpful discussions, Azadeh T. Kia for document proofreading, and Dr. David M. Mosser for critical reading of the manuscript before submission.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Edwards JL, Apicella MA. The molecular mechanisms used by Neisseria gonorrhoeae to initiate infection differ between men and women. Clin Microbiol Rev. 2004;17:965–981s. doi: 10.1128/CMR.17.4.965-981.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merz AJ, Rifenbery DB, Arvidson CG, So M. Traversal of a polarized epithelium by pathogenic Neisseriae: facilitation by Type IV pili and maintenance of epithelial barrier function. Mol Med. 1996;2:745–754. [PMC free article] [PubMed] [Google Scholar]
- 3.Griffiss JM, Lammel CJ, Wang J, Dekker NP, Brooks GF. Neisseria gonorrhoeae coordinately uses pili and Opa to activate HEC-1- B cell microvilli, which causes engulfment of the gonococci. Infect Immun. 1999;67:3469–3480. doi: 10.1128/iai.67.7.3469-3480.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harvey HA, Ketterer MR, Preston A, Lubaroff D, Williams R, Apicella MA. Ultrastructural analysis of primary human urethral epithelial cultures infected with Neisseria gonorrhoeae. Infect Immun. 1997;65:2420–2417. doi: 10.1128/iai.65.6.2420-2427.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGee ZA, Stephens DS, Hoffman LH, Schlech WF, III, Horn RG. Mechanisms of mucosal invasion by pathogenic Neisseria. Rev Infect Dis. 1983;5:S708–S714. doi: 10.1093/clinids/5.supplement_4.s708. [DOI] [PubMed] [Google Scholar]
- 6.Rudel T, van Putten JP, Gibbs CP, Haas R, Meyer TF. Interaction of two variable proteins (PilE and PilC) required for pilus-mediated adherence of Neisseria gonorrhoeae to human epithelial cells. Mol Microbiol. 1992;6:3439–3450. doi: 10.1111/j.1365-2958.1992.tb02211.x. [DOI] [PubMed] [Google Scholar]
- 7.Swanson J. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J Exp Med. 1973;137:571–589. doi: 10.1084/jem.137.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ward ME, Watt PJ. Adherence of Neisseria gonorrhoeae to urethral mucosal cells: An electron microscopic study of human gonorrhea. J Infect Dis. 1972;126:601–605. doi: 10.1093/infdis/126.6.601. [DOI] [PubMed] [Google Scholar]
- 9.Merz AJ, So M. Attachment of piliated, Opa- and Opc- gonococci and meningococci to epithelial cells elicits cortical actin rearrangements and clustering of tyrosine-phosphorylated proteins. Infect Immun. 1997;65:4341–4349. doi: 10.1128/iai.65.10.4341-4349.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw JH, Falkow S. Model for invasion of human tissue culture cells by Neisseria gonorrhoeae. Infect Immun. 1988;56:1625–1632. doi: 10.1128/iai.56.6.1625-1632.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isberg RR, Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature. 1985;317:262–264. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- 12.White LA, Kellogg DS., Jr Neisseria gonorrhoeae identification in direct smears by a fluorescent antibody counterstain method. Appl Microbiol. 1965;13:171–174. doi: 10.1128/am.13.2.171-174.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patrone JB, Bish SE, Stein DC. TNF-alpha-independent IL-8 expression: alterations in bacterial challenge dose cause differential human monocytic cytokine response. J Immunol. 2006;177:1314–1322. doi: 10.4049/jimmunol.177.2.1314. [DOI] [PubMed] [Google Scholar]
- 14.Stein DC, Young FE, Tenover FC, Clark VL. Characterization of a chimeric β-lactamase plasmid of Neisseria gonorrhoeae which can function in Escherichia coli. Mol Gen Genet. 1983;189:77–84. doi: 10.1007/BF00326058. [DOI] [PubMed] [Google Scholar]
- 15.Sandlin RC, Apicella MA, Stein DC. Cloning of a gonococcal DNA sequence that complements the lipooligosaccharide defects of Neisseria gonorrhoeae 1291d and 1291e. Infect Immun. 1993;61:3360–3368. doi: 10.1128/iai.61.8.3360-3368.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Callaghan CHO’, Morris A, Kirby SM, Shingler AH. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972;1:283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merz AJ, So M. Interactions of pathogenic Neisseriae with epithelial cell membranes. Annu Rev Cell Dev Biol. 2000;16:423–457. doi: 10.1146/annurev.cellbio.16.1.423. [DOI] [PubMed] [Google Scholar]
- 18.Minor SY, Banerjee A, Gotschlich EC. Effect of alpha-oligosaccharide phenotype of Neisseria gonorrhoeae strain MS11 on invasion of Chang conjunctival, HEC-1-B endometrial, and ME-180 cervical cells. Infect Immun. 2000;68:6526–6534. doi: 10.1128/iai.68.12.6526-6534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swanson KV, Jarvis GA, Brooks GF, Barham BJ, Cooper MD, Griffiss JM. CEACAM is not necessary for Neisseria gonorrhoeae to adhere to and invade female genital epithelial cells. Cell Microbiol. 2001;3:681–691. doi: 10.1046/j.1462-5822.2001.00147.x. [DOI] [PubMed] [Google Scholar]
- 20.Weel JF, Hopman CT, van Putten JP. Stable expression of lipooligosaccharide antigens during attachment, internalization, and intracellular processing of Neisseria gonorrhoeae in infected epithelial cells. Infect Immun. 1989;57:3395–3402. doi: 10.1128/iai.57.11.3395-3402.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merz AJ, So M, Sheetz MP. Pilus retraction powers bacterial twitching motility. Nature. 2000;407:98–102. doi: 10.1038/35024105. [DOI] [PubMed] [Google Scholar]
- 22.Lee SW, Higashi DL, Snyder A, Merz AJ, Potter L, So M. PilT is required for PI(3,4,5)P3-mediated crosstalk between Neisseria gonorrhoeae and epithelial cells. Cell Microbiol. 2005;7:1271–1284. doi: 10.1111/j.1462-5822.2005.00551.x. [DOI] [PubMed] [Google Scholar]
- 23.Deghmane AE, Larribe M, Giorgini D, Sabino D, Taha MK. Differential expression of genes that harbor a common regulatory element in Neisseria meningitidis upon contact with target cells. Infect Immun. 2003;71:2897–2901. doi: 10.1128/IAI.71.5.2897-2901.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deghmane AE, Petit S, Topilko A, Pereira Y, Giorgini D, Larribe M, Taha MK. Intimate adhesion of Neisseria meningitidis to human epithelial cells is under the control of the crgA gene, a novel LysR-type transcriptional regulator. EMBO J. 2000;19:1068–1078. doi: 10.1093/emboj/19.5.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morelle S, Carbonnelle E, Nassif X. The REP2 repeats of the genome of Neisseria meningitidis are associated with genes coordinately regulated during bacterial cell interaction. J Bacteriol. 2003;185:2618–2627. doi: 10.1128/JB.185.8.2618-2627.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGee ZA, Johnson AP, Taylor-Robinson D. Human fallopian tubes in organ culture: preparation, maintenance, and quantitation of damage by pathogenic microorganisms. Infect Immun. 1976;13:608–618. doi: 10.1128/iai.13.2.608-618.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pils S, Schmitter T, Neske F, Hauck CR. Quantification of bacterial invasion into adherent cells by flow cytometry. J Microbiol Methods. 2006;65:301–310. doi: 10.1016/j.mimet.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Mosleh IM, Boxberger HJ, Sessler MJ, Meyer TF. Experimental infection of native human urethral tissue with Neisseria gonorrhoeae: adhesion, invasion, intracellular fate, exocytosis, and passage through a stratified epithelium. Infect Immun. 1997;65:3391–3398. doi: 10.1128/iai.65.8.3391-3398.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams JM, Chen GC, Zhu L, Rest RF. Using the yeast two-hybrid system to identify human epithelial cell proteins that bind gonococcal Opa proteins: intracellular gonococci bind pyruvate kinase via their Opa proteins and require host pyruvate for growth. Mol Microbiol. 1998;27:171–186. doi: 10.1046/j.1365-2958.1998.00670.x. [DOI] [PubMed] [Google Scholar]