Abstract
STAT3 is constitutively activated in several cancers, including prostate cancer, and is therefore, a potential target for cancer therapy. DU-145 prostate cancer cells were stably co-transfected with STAT3 reporter and puromycin resistant plasmids to create a stable STAT3 reporter cell line that can be used for high throughput screening of STAT3 modulators. The applicability of this cell line was tested with two known activators and inhibitors of STAT3. As expected, EGF and IL-6 increased STAT3 reporter activity and enhanced the nuclear localization of phosphorylated STAT3 (pSTAT3); whereas Cucurbitacin I and AG490 decreased STAT3 reporter activity dose and time-dependently and reduced the localization of pSTAT3 in the nuclei of prostate cancer cells. Given the importance of STAT3 in cancer initiation and progression, the development of a stable STAT3 reporter cell line in prostate cancer cells provides a rapid, sensitive, and cost effective method for the screening of potential STAT3 modulators.
Keywords: STAT3 reporter cell line, prostate cancer, STAT3 activators, STAT3 inhibitors, high throughput screening
Introduction
Signal transducers and activators of transcription (STATs) are a seven member family class of transcription factors that are involved in physiological functions such as immune response, proliferation, apoptosis, and cell survival in normal cells [1–3]. Some of the STAT proteins are upregulated in cancer cells. In particular, STAT3 is constitutively activated in several cancers such as breast, lung, leukemias-lymphomas, and prostate [4, 5]. The constitutive activation of STAT3 may be caused by genetic mutations such as the overexpression of human epidermal growth factor receptor 2 (EGFR2/HER2) or EGFR1/HER1 [6, 7]. STAT3 can contribute to tumor growth by initiating the cell cycle, preventing apoptosis, and upregulating oncogenes such as c-Myc and Bcl-X [8]. Furthermore, STAT3 has recently been demonstrated to augment prostate cancer metastasis by promoting prostate cancer cell migration [9].
STAT3 can be activated by cytokines such as interleukin-6 (IL-6) or interferons. IL-6 binds to cell surface receptor tyrosine kinases that are coupled with and activate Janus kinases (JAKs). The JAKs then phosphorylate the tyrosine 705 residue in the transactivation domain of the STAT3 protein [8]. The phosphorylation of tyrosine 705 induces STAT3 dimerization, nuclear translocation, and DNA binding to STAT3 response elements. Serine at the 727th residue in the same domain must also be phosphorylated for complete transcriptional activity [10]. IL-6 activation of STAT3 has been implicated in prostate cancer progression [5, 11]. In addition to cytokines, growth factors such as epidermal growth factor (EGF) and platelet derived growth factor (PDGF) can also activate STAT3 [10, 12].
Conversely, cytokines can inhibit STAT3 signaling. Cytokine inducible genes constituting the suppressors of cytokine signaling (SOCS) protein family can bind to and inhibit JAKs, thus repressing STAT3 activation [13]. The protein inhibitors of activated STATs (PIAS) are a second group of proteins that decrease STAT3 signaling by preventing STAT3 DNA binding activity [14]. Chemical inhibitors of JAK and STAT3 have also been shown to reduce STAT3 activation and the survival of different cancer cells [5, 15]. AG490 is a chemical inhibitor of JAK2 and it can suppress STAT3 signaling by inhibiting the DNA binding activity of STAT3 [16–18]. Cucurbitacin I is another inhibitor of both JAK2 and STAT3. Cucurbitacin I inhibits the tyrosine phosphorylation of JAK2 and STAT3, thereby preventing STAT3 DNA binding and STAT3 mediated gene transcription [19].
Since STAT3 is associated with various cancers, positive and negative regulators of STAT3 signaling could play a pivotal role in controlling tumorigenesis. Positive regulators of STAT3 could provide valuable information about the activation of STAT3, which could identify new targets for the design of new inhibitors. For example, inhibitors for tyrosine or serine kinases that activate STAT3 can be produced. The search for negative regulators of STAT3 activation is imperative since STAT3 has been confirmed as a valid target for the treatment of cancers [10, 16, 20]. The inhibition of constitutively active STAT3 by protein inhibitors has been demonstrated to decrease tumor cell proliferation and promote apoptosis both in vitro and in vivo [4, 21, 22]. However, the inhibition of STAT3 in normal cells results in growth arrest but not apoptosis, indicating that STAT3 may be targeted for preferential cancer cell eradication [23, 24]. The discovery of STAT3 modulators can be greatly advanced with high throughput screening of accessible and multiple compound libraries. Using a chemiluminescent reporter activity assay as the method of choice for high throughput screening would provide an efficient and quick turnaround of screening data. To this end, we have developed a stable STAT3 reporter prostate cancer cell line for the screening of potential STAT3 activators and inhibitors which could eventually identify new STAT3 inhibitors for future clinical use.
Materials and Methods
Cell culture
DU-145 human prostate cancer cells were purchased from the American Type Cell Culture Collection (Manassas, VA). The cells were grown in complete growth medium (Improved Minimum Essential Medium (IMEM) without phenol red; Invitrogen; Carlsbad, CA) supplemented with 10% fetal bovine serum (Quality Biological; Gaithersburg, MD), 100 units/ml penicillin G sodium, 100 μg/ml streptomycin sulfate, and 2 mM glutamine (Sigma Chemicals; St. Louis, MO) in the presence of 5% CO2 at 37°C.
Development of stable STAT3 reporter cell line
DU-145 cells were co-transfected with 200 ng of plasmid DNA containing three STAT3 response elements upstream of a thymidine kinase promoter and a luciferase reporter (Clontech; Mountain View, CA) and 20 ng of plasmid DNA containing a puromycin resistant gene (Clontech) using GeneJammer transfection reagent (Stratagene; La Jolla, CA). Two days after the transfection, the cells were replated and continually grown in the presence of 1 μg/ml of puromycin. After four weeks, 12 puromycin resistant colonies were isolated using cell cloning cylinders and cultured in puromycin containing media. The STAT3 luciferase activity of each colony was measured to verify the stable transfection and to re-establish that the clonal population arose from a single cell.
STAT3 reporter activity assays
DU-145 STAT3 reporter cells were seeded in 96-well plates (2×103 cells/well) in triplicate. The following day, the cells were treated with Cucurbitacin I and AG490 at varying concentrations and times. The cells treated with EGF and IL-6 were serum starved for three days prior to treatment. Luciferase activity was measured in cell lysates by a microplate luminometer (Harta Instruments, Inc; Gaithersburg, MD) using the Firefly Luciferase Assay kit (Biotium, Inc; Hayward, CA) according to the manufacturer’s protocol.
Immunofluorescence staining
DU-145 cells stably expressing the STAT3 reporter construct were plated onto ECL-coated chamber slides. The cells were fixed in methanol, air-dried, and re-hydrated with phosphate buffered saline (PBS). The cells were blocked with 0.2% crystalline grade BSA and subsequently incubated with the primary antibody (pSTAT3 Y705, 1:100 dilution, Cell Signaling Technology; Danvers, MA) overnight at 4°C. After three washes with PBS, the cells were incubated with Alexa Fluor 488 (4 μg/ml) conjugated secondary antibody (Molecular Probes, Invitrogen; Carlsbad, CA) for one hour. The cells were washed three more times with PBS and subsequently incubated with 250 ng/ml of propidium iodide (PI) (Biotium, Inc.) for 5 min. The cells were washed once more with PBS, mounted with 50% glycerol, and viewed under a fluorescent microscope (ZEISS AxioPlan2 Imaging System, Jena, Germany). Images were captured at the same magnification (20x) and then imported into Adobe Photoshop.
Statistical analysis
Statistical analyses on all data derived from at least three independent experiments were conducted using Prism 3 GraphPad software. Data values were presented as mean ± SEM. The significance level was calculated using the one-way Analysis of Variance (ANOVA) followed by the Dunnett post-test with an assigned confidence interval of 95%. A p-value < 0.05 was considered significant.
Results & Discussion
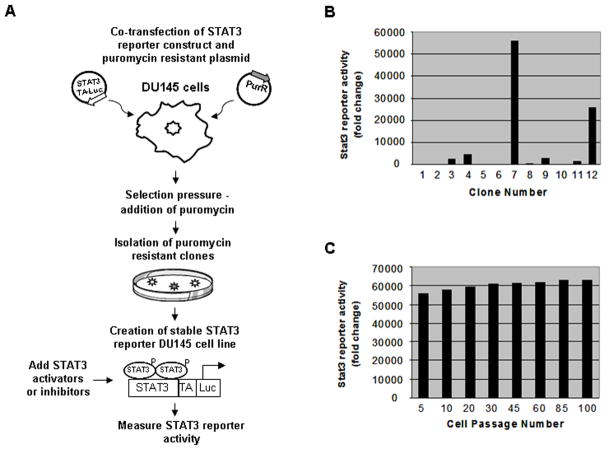
There is an urgent need for the development of high throughput screening for inhibitors of various oncogenic factors. We are in an era when hundreds of thousands of small molecule libraries are available to screen possible inhibitors for numerous targets. These small molecule libraries cannot be screened without the development of appropriate high throughput screening of cell lines with various cancer backgrounds. Keeping that in mind, we developed a stable STAT3 reporter cell line where DU-145 human prostate cancer cells were stably co-transfected with a puromycin resistant plasmid and a STAT3 plasmid containing three STAT3 response elements upstream of a thymidine kinase promoter and a luciferase reporter. The stable transfection protocol is outlined in Figure 1A. Information pertaining to the clones is detailed in Figure 1B. Out of the 12 clones, clone number 7 had the highest STAT3 reporter activity and was consequently retained as the stable STAT3 reporter cell line. The STAT3 reporter activity in clone number 7 remained stable through 100 cell passages (Fig. 1C). Clones number 1, 2, 5, 6, 8 and 10 had minimal or no STAT3 reporter activity indicating that only the puromycin resistant plasmid or very few copies of STAT3 reporter plasmid were integrated into the cells’ genome. All subsequent experiments were performed with STAT3 reporter cells from clone number 7 between passages 45 to 60.
Fig. 1.
The establishment of a STAT3 reporter prostate cancer cell line. (A) Schematic of the stable transfection protocol. DU-145 cells were co-transfected with plasmids containing a STAT3 reporter and a puromycin resistant gene (purR). The transfected cells were continually grown in selective media containing puromycin (1 μg/ml). Four weeks later, colonies were isolated to determine the STAT3 reporter activity of the stable transfection. (B) Selection of STAT3 reporter cell line. STAT3 reporter activity of twelve clones was assessed at passage number 5. The clone with the highest STAT3 reporter activity (number 7) was designated as the stable STAT3 reporter cell line. (C) Graph showing STAT3 reporter activity is stabilized in clone number 7 through 100 cell passages.
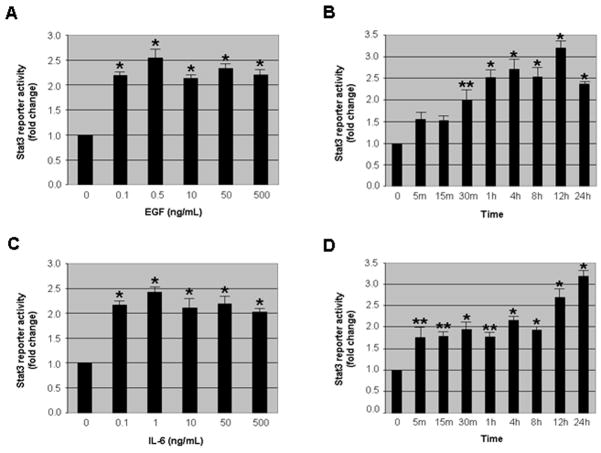
To confirm that the stably transfected cells are functionally active, the DU-145 STAT3 reporter cells were treated with two established STAT3 activators, EGF and IL-6. First, dose curves with various concentrations were performed (Fig. 2A and C). Both activators increased STAT3 reporter activity at least 2-fold or higher and then plateaued. The maximum increases in STAT3 reporter activity for EGF and IL-6 were observed with 0.5 ng/ml and 1 ng/ml, respectively. These concentrations are at least 10-fold less than the concentrations used in previously published studies using Western blot and EMSA analyses [25, 26]. This result suggests that the STAT3 reporter activity assay is more sensitive compared to other assays. However, different cell lines and experimental conditions could account for the concentration differences. Next, using 0.5 ng/ml of EGF and 1 ng/ml of IL-6, we examined the time course of STAT3 reporter activation. As observed in Figures 2B and 2D, a significant increase in STAT3 reporter activity was detected within 5 minutes and 30 minutes for IL-6 and EGF, respectively, with a maximum increase of 3.2-fold observed at 12 and 24 hours for EGF and IL-6, respectively. The dose curves and time courses for the activators follow the expected effects for growth factors and cytokines, as there is a dose-dependent increase in STAT3 reporter activity and then saturation. Over a longer period of treatment, STAT3 reporter activity declined after reaching a peak, which could be as a result of EGF and IL-6 receptor desensitization and degradation (data not shown). Since DU-145 cells synthesize IL-6 [16], we observed a high level of STAT3 reporter activity in normal growth medium without any addition of STAT3 activators, conferring an advantage for the identification of STAT3 inhibitors with high throughput screening because it will not be necessary to induce STAT3 activation in these cells prior to the addition of STAT3 inhibitors.
Fig. 2.
The stable STAT3 reporter prostate cancer cell line is functionally active. (A,C) The evaluation of STAT3 reporter activity with various concentrations of EGF and IL-6. STAT3 reporter cells were treated with varying concentrations of EGF and IL-6 for 30 minutes and then STAT3 reporter activity was measured. All concentrations of EGF and IL-6 increased STAT3 reporter activity significantly (p<0.01). (B,D) The assessment of treatment time for STAT3 reporter activity with STAT3 activators. The STAT3 reporter cells were treated with 0.5 ng/ml of EGF and 1 ng/ml of IL-6 for 5 minutes to 24 hours. A significant (p<0.05 or p<0.01) increase in STAT3 reporter activity was observed with both EGF and IL-6 treatment. Reporter activity in control samples was considered as 1.0. Columns, mean of three independent experiments; bars, standard error (SE). **, p<0.05, *, p<0.01, significantly different from control.
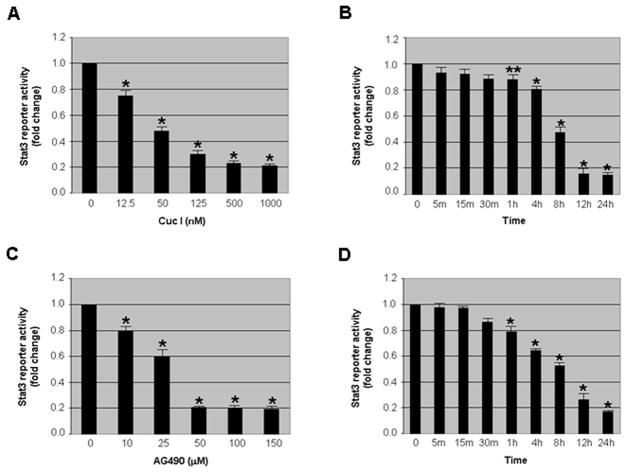
To confirm that our STAT3 reporter cell line can be used to detect STAT3 inhibitors, we used two known STAT3 inhibitors, Cucurbitacin I and AG490, in our assays. As shown in Figures 3A and 3C, there was a dose-dependent decrease in STAT3 reporter activity with both Cucurbitacin I and AG490. Approximately 50% of STAT3 reporter activity was decreased with 50 nM of Cucurbitacin I and 25 μM of AG490. A time course assay utilizing the lowest effective concentrations, 500 nM of Cuc I and 50 μM of AG490, showed a time-dependent decrease for both inhibitors with the maximum decrease (~80%) in STAT3 reporter activity at 24 hours (Fig. 3B and D). A treatment time beyond 24 hours with Cucurbitacin I and AG490 resulted in cell death (data not shown). However, there was no toxicity (cell death) with either Cucurbitacin I or AG490 with 24 hours of treatment, implicating that the inhibition of STAT3 reporter activity was not associated with cell death. The concentrations and length of treatment for Cucurbitacin I and AG490 are consistent with previously published studies [27, 28], suggesting that our newly established STAT3 reporter cell line can be used for high throughput screening of STAT3 inhibitors.
Fig. 3.
STAT3 inhibitors decrease STAT3 reporter activity in the stable cell line. (A,C) The evaluation of STAT3 reporter activity with various concentrations of STAT3 inhibitors, Cucurbitacin I (Cuc I) and AG490. STAT3 reporter cells were treated with various concentrations of Cuc I and AG490 for 24 hours and then STAT3 reporter activity was assessed. A dose-dependent decrease in STAT3 reporter activity was observed with both Cucurbitacin I (Cuc I) and AG490. (B,D) The assessment of treatment time for STAT3 reporter activity with STAT3 inhibitors. The cells were treated with the 500 nM of Cuc I and 50 μM of AG490 from 5 minutes to 24 hours. There is a significant (p<0.05 or p<0.01) decrease in STAT3 reporter activity that was observed as early as 1 hour and maintained up to 24 hours in both Cuc I and AG490 treatment. Reporter activity in control samples was considered as 1.0. Columns, mean; bars, SE. **, p<0.05, *, p<0.01, significantly different from control.
Although we demonstrated that STAT3 activators and inhibitors can modulate STAT3 reporter activity in prostate cancer cells, we have not examined whether STAT3 activators and inhibitors phosphorylate STAT3 and localize phosphorylated STAT3 in the nuclei of the STAT3 reporter cells. To this end, we performed immunofluorescence staining of phosphorylated STAT3 Y705 (pSTAT3 Y705) in stable STAT3 reporter DU-145 cells. pSTAT3 Y705 is generally localized in the nuclei of the cells with minimal pSTAT3 in the cytoplasm. The nuclear localization of pSTAT3 Y705 increased with EGF and IL-6 treatment (1 ng/ml) for 24 hours (Fig. 4A). In contrast, the STAT3 inhibitors, Cucurbitacin I (500 nM) and AG490 (50 μM) reduced nuclear pSTAT3 Y705 staining within 24 hours (Fig. 4B). These results demonstrate that EGF and IL-6 increased activated STAT3 in the nuclei of prostate cancer cells and the inhibitors had the opposite effect. Thus, the immunofluorescence data corroborates with the STAT3 reporter activity of the stable STAT3 reporter prostate cancer cell line.
Fig. 4.
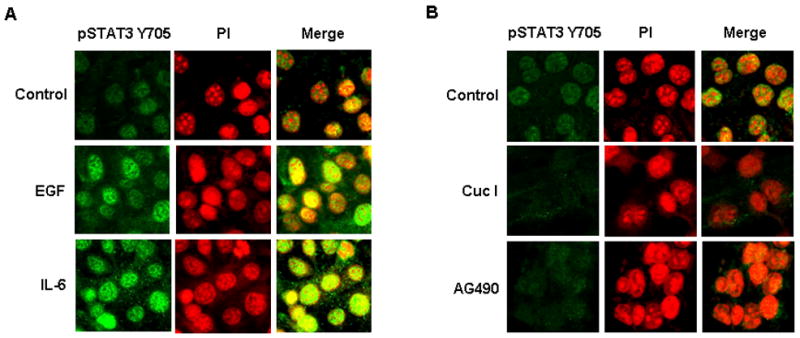
The activation of phosphorylated STAT3 Y705 (pSTAT3 Y705) is altered by STAT3 activators and inhibitors. (A) Immunofluorescence staining of pSTAT3 Y705 in DU-145 cells stably expressing the STAT3 reporter construct after treatment with EGF and IL-6. Both activators of STAT3 increased pSTAT3 Y705 in the nuclei of STAT3 reporter stable DU-145 cells. (B) Immunofluorescence of pSTAT3 Y705 in STAT3 reporter cells treated with Cuc I and AG490. STAT3 inhibitors decreased activation and nuclear localization of pSTAT3 Y705.
The development of STAT3 inhibitors is an area of intense interest due to the important role STAT3 has in carcinogenesis. Currently, another analog of Cucurbitacin, Cucurbitacin Q, is the only selective STAT3 signaling inhibitor available [3]. Small molecule inhibitors of STAT3 without their full elucidated effects have been developed in the last few years and more research in peptidomimetics is necessary [29, 30]. Activators of STAT3 are also of importance as all activators of STAT3 have yet to be elucidated. The urgency to identify STAT3 regulators facilitates the necessity for a quick and efficient method of analyzing potential STAT3 activators and inhibitors. Luciferase reporter assays are a reliable, fast, sensitive, quantitative, and cost effective approach to determining the level of STAT3 activation by modulators and should be used as the first method to screen potential STAT3 activators and inhibitors. The development of a stable STAT3 reporter cell line streamlines the screening process, eliminating the transient transfection step, and can be used for any scale of screening from low to high throughput.
Acknowledgments
This work was supported by NIH grant R01 DK060875 (to PPB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Horvath CM, Wen Z, Darnell JE., Jr A STAT protein domain that determines DNA sequence recognition suggests a novel DNA-binding domain. Genes Dev. 1995;9:984–994. doi: 10.1101/gad.9.8.984. [DOI] [PubMed] [Google Scholar]
- 2.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 3.Sun J, Blaskovich MA, Jove R, Livingston SK, Coppola D, Sebti SM. Cucurbitacin Q: a selective STAT3 activation inhibitor with potent antitumor activity. Oncogene. 2005;24:3236–3245. doi: 10.1038/sj.onc.1208470. [DOI] [PubMed] [Google Scholar]
- 4.Ni Z, Lou W, Leman ES, Gao AC. Inhibition of constitutively activated Stat3 signaling pathway suppresses growth of prostate cancer cells. Cancer Res. 2000;60:1225–1228. [PubMed] [Google Scholar]
- 5.Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res. 2002;8:945–954. [PubMed] [Google Scholar]
- 6.Fernandes A, Hamburger AW, Gerwin BI. ErbB-2 kinase is required for constitutive stat 3 activation in malignant human lung epithelial cells. Int J Cancer. 1999;83:564–570. doi: 10.1002/(sici)1097-0215(19991112)83:4<564::aid-ijc20>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 7.Berclaz G, Altermatt HJ, Rohrbach V, Siragusa A, Dreher E, Smith PD. EGFR dependent expression of STAT3 (but not STAT1) in breast cancer. Int J Oncol. 2001;19:1155–1160. doi: 10.3892/ijo.19.6.1155. [DOI] [PubMed] [Google Scholar]
- 8.Calo V, Migliavacca M, Bazan V, Macaluso M, Buscemi M, Gebbia N, Russo A. STAT proteins: from normal control of cellular events to tumorigenesis. J Cell Physiol. 2003;197:157–168. doi: 10.1002/jcp.10364. [DOI] [PubMed] [Google Scholar]
- 9.Abdulghani J, Gu L, Dagvadorj A, Lutz J, Leiby B, Bonuccelli G, Lisanti MP, Zellweger T, Alanen K, Mirtti T, Visakorpi T, Bubendorf L, Nevalainen MT. Stat3 promotes metastatic progression of prostate cancer. Am J Pathol. 2008;172:1717–1728. doi: 10.2353/ajpath.2008.071054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 11.Chiu JJ, Sgagias MK, Cowan KH. Interleukin 6 acts as a paracrine growth factor in human mammary carcinoma cell lines. Clin Cancer Res. 1996;2:215–221. [PubMed] [Google Scholar]
- 12.Turkson J, Bowman T, Garcia R, Caldenhoven E, De Groot RP, Jove R. Stat3 activation by Src induces specific gene regulation and is required for cell transformation. Mol Cell Biol. 1998;18:2545–2552. doi: 10.1128/mcb.18.5.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Starr R, Hilton DJ. Negative regulation of the JAK/STAT pathway. Bioessays. 1999;21:47–52. doi: 10.1002/(SICI)1521-1878(199901)21:1<47::AID-BIES6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 14.Chung CD, Liao J, Liu B, Rao X, Jay P, Berta P, Shuai K. Specific inhibition of Stat3 signal transduction by PIAS3. Science. 1997;278:1803–1805. doi: 10.1126/science.278.5344.1803. [DOI] [PubMed] [Google Scholar]
- 15.Garcia R, Bowman TL, Niu G, Yu H, Minton S, Muro-Cacho CA, Cox CE, Falcone R, Fairclough R, Parsons S, Laudano A, Gazit A, Levitzki A, Kraker A, Jove R. Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene. 2001;20:2499–2513. doi: 10.1038/sj.onc.1204349. [DOI] [PubMed] [Google Scholar]
- 16.Barton BE, Karras JG, Murphy TF, Barton A, Huang HF. Signal transducer and activator of transcription 3 (STAT3) activation in prostate cancer: Direct STAT3 inhibition induces apoptosis in prostate cancer lines. Mol Cancer Ther. 2004;3:11–20. [PubMed] [Google Scholar]
- 17.Seidel HM, Lamb P, Rosen J. Pharmaceutical intervention in the JAK/STAT signaling pathway. Oncogene. 2000;19:2645–2656. doi: 10.1038/sj.onc.1203550. [DOI] [PubMed] [Google Scholar]
- 18.Huang C, Cao J, Huang KJ, Zhang F, Jiang T, Zhu L, Qiu ZJ. Inhibition of STAT3 activity with AG490 decreases the invasion of human pancreatic cancer cells in vitro. Cancer Sci. 2006;97:1417–1423. doi: 10.1111/j.1349-7006.2006.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blaskovich MA, Sun J, Cantor A, Turkson J, Jove R, Sebti SM. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res. 2003;63:1270–1279. [PubMed] [Google Scholar]
- 20.Turkson J, Jove R. STAT proteins: novel molecular targets for cancer drug discovery. Oncogene. 2000;19:6613–6626. doi: 10.1038/sj.onc.1204086. [DOI] [PubMed] [Google Scholar]
- 21.Lin TS, Mahajan S, Frank DA. STAT signaling in the pathogenesis and treatment of leukemias. Oncogene. 2000;19:2496–2504. doi: 10.1038/sj.onc.1203486. [DOI] [PubMed] [Google Scholar]
- 22.Lee SO, Lou W, Qureshi KM, Mehraein-Ghomi F, Trump DL, Gao AC. RNA interference targeting Stat3 inhibits growth and induces apoptosis of human prostate cancer cells. Prostate. 2004;60:303–309. doi: 10.1002/pros.20072. [DOI] [PubMed] [Google Scholar]
- 23.Bowman T, Broome MA, Sinibaldi D, Wharton W, Pledger WJ, Sedivy JM, Irby R, Yeatman T, Courtneidge SA, Jove R. Stat3-mediated Myc expression is required for Src transformation and PDGF-induced mitogenesis. Proc Natl Acad Sci U S A. 2001;98:7319–7324. doi: 10.1073/pnas.131568898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niu G, Heller R, Catlett-Falcone R, Coppola D, Jaroszeski M, Dalton W, Jove R, Yu H. Gene therapy with dominant-negative Stat3 suppresses growth of the murine melanoma B16 tumor in vivo. Cancer Res. 1999;59:5059–5063. [PubMed] [Google Scholar]
- 25.Tyagi A, Agarwal R, Agarwal C. Grape seed extract inhibits EGF-induced and constitutively active mitogenic signaling but activates JNK in human prostate carcinoma DU145 cells: possible role in antiproliferation and apoptosis. Oncogene. 2003;22:1302–1316. doi: 10.1038/sj.onc.1206265. [DOI] [PubMed] [Google Scholar]
- 26.Mora LB, Buettner R, Seigne J, Diaz J, Ahmad N, Garcia R, Bowman T, Falcone R, Fairclough R, Cantor A, Muro-Cacho C, Livingston S, Karras J, Pow-Sang J, Jove R. Constitutive activation of Stat3 in human prostate tumors and cell lines: direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells. Cancer Res. 2002;62:6659–6666. [PubMed] [Google Scholar]
- 27.Qureshi K, Lee S, Lou W, Trump D, Gao A. Targeting stat3 signaling in human prostate cancer cells using tyrosine kinase inhibitors. Journal of Clinical Oncology; 2004 ASCO Annual Meeting Proceedings (Post-Meeting Edition); 2004. p. 3138. [Google Scholar]
- 28.Ueda T, Bruchovsky N, Sadar MD. Activation of the androgen receptor N-terminal domain by interleukin-6 via MAPK and STAT3 signal transduction pathways. J Biol Chem. 2002;277:7076–7085. doi: 10.1074/jbc.M108255200. [DOI] [PubMed] [Google Scholar]
- 29.Schust J, Sperl B, Hollis A, Mayer TU, Berg T. Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem Biol. 2006;13:1235–1242. doi: 10.1016/j.chembiol.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 30.Turkson J, Ryan D, Kim JS, Zhang Y, Chen Z, Haura E, Laudano A, Sebti S, Hamilton AD, Jove R. Phosphotyrosyl peptides block Stat3-mediated DNA binding activity, gene regulation, and cell transformation. J Biol Chem. 2001;276:45443–45455. doi: 10.1074/jbc.M107527200. [DOI] [PubMed] [Google Scholar]