Abstract
We tested the hypothesis that low vitamin D receptor (VDR) level causes intestinal vitamin D resistance and intestinal calcium (Ca) malabsorption. To do so, we examined vitamin D regulated duodenal Ca absorption and gene expression [transient receptor potential channel, vallinoid subfamily member 6 (TRPV6), 24-hydroxylase, calbindin D9k (CaBP) mRNA, and CaBP protein] in wild-type mice and mice with reduced tissue VDR levels [i.e. heterozygotes for the VDR gene knockout (HT)]. Induction of 24-hydroxylase mRNA levels by 1,25 dihydroxyvitamin D3 [1,25(OH)2 D3] injection was significantly reduced in the duodenum and kidney of HT mice in both time-course and dose-response experiments. TRPV6 and CaBP mRNA levels in duodenum were significantly induced after 1,25(OH)2 D3 injection, but there was no difference in response between wild-type and HT mice. Feeding a low-calcium diet for 1 wk increased plasma PTH, renal 1α-hydroxylase (CYP27B1) mRNA level, and plasma 1,25(OH)2 D3, and this response was greater in HT mice (by 88, 55, and 37% higher, respectively). In contrast, duodenal TRPV6 and CaBP mRNA were not higher in HT mice fed the low-calcium diet. However, the response of duodenal Ca absorption and CaBP protein to increasing 1,25(OH)2 D3 levels was blunted by 40% in HT mice. Our data show that low VDR levels lead to resistance of intestinal Ca absorption to 1,25(OH)2 D3, and this resistance may be due to a role for the VDR (and VDR level) in the translation of CaBP.
LOW EFFICIENCY OF intestinal calcium absorption is a risk factor for hip fracture in women (1), and low calcium absorption efficiency has been observed in postmenopausal women with vertebral and peripheral fractures (2). As such, the study of factors that regulate intestinal calcium absorption has relevance to the prevention of osteoporosis. Intestinal calcium absorption is comprised of a nonregulated component that is presumably diffusional and paracellular as well as a regulated, transcellular component (3). Transcellular intestinal calcium absorption is regulated primarily by 1,25 dihydroxyvitamin D [1,25(OH)2 D] via transcriptional activation of genes in a process dependent on the classical nuclear vitamin D receptor (VDR) (4, 5). Four proteins have been proposed to be essential for transcellular calcium absorption: transient receptor potential channel, vallinoid subfamily member 6 (TRPV6) (a.k.a. CaT1 or ECAC2), a apical membrane calcium channel that mediates calcium entry into enterocytes (6, 7); calbindin D9k, an intracellular calcium binding protein that buffers intracellular calcium levels during calcium absorption and facilitates calcium movement through the cytoplasm (3, 8, 9); and two basolateral membrane proteins that mediate extrusion of calcium from cells: plasma membrane calcium ATPase-1b, an ATP-dependent calcium pump (10) and a sodium-calcium exchanger (11). The level of each of these proteins or their transcripts is known to be increased by 1,25 dihydroxyvitamin D3 [1,25(OH)2 D3] in the intestine (11-13).
Several studies show that the efficiency of intestinal calcium absorption falls with age (14-17) and that this may be due to the development of intestinal resistance to the action of 1,25(OH)2 D3 (18-20). In addition to aging, low estrogen status also causes intestinal vitamin D resistance (21). The molecular mechanism of this age- or estrogen deficiency-induced intestinal resistance to 1,25(OH)2 D3 is not clear, but several groups have suggested that a 30-50% reduction in intestinal VDR levels is responsible. Studies show that intestinal VDR content decreases with age in rats and humans (15, 22). Similarly, ovariectomy reduces intestinal VDR levels in rats (23) whereas estrogen-repletion restores intestinal VDR levels to normal in ovariectomized rats (24). We have previously shown that Caco-2 cells genetically modified to express higher VDR levels are more responsive to 1,25(OH)2 D3; both 1,25(OH)2 D3-regulated transcellular calcium absorption and gene expression are higher in cells with elevated VDR content (25). However, whereas these studies are consistent with the hypothesis that low VDR level limits intestinal responsiveness to 1,25(OH)2 D3, formal testing of this hypothesis in the complex setting of whole-body physiology has not been attempted.
In the last decade, several groups have independently generated VDR knockout mice (VDR KO) by targeted disruption of VDR gene (4, 26-28). VDR KO mice exhibit a phenotype of vitamin D-resistant rickets and have significantly reduced calcium absorption, compared with wild-type (WT) mice (4, 5). Mice lacking one VDR allele [heterozygous (HT)] have only half the intestinal VDR content of WT mice, but when raised on a normal, chow-type diet, HT mice are phenotypically normal (26). With their reduced tissue VDR levels, these mice provide a unique opportunity to assess the impact of low VDR level on the ability of the intestine to respond to increased circulating levels of 1,25(OH)2 D3, resulting from injection or dietary calcium manipulation. Thus, the aim of our study was to test the role VDR level plays in the intestinal response to 1,25(OH)2 D3 in WT and HT mice.
Materials and Methods
Chemicals
All the chemicals were purchased from Sigma Chemical Company (St. Louis, MO) unless otherwise specified below.
Animals
Normal healthy wild-type and VDR heterozygous mice were obtained from our breeding colony of VDR heterozygous mice and selected based on genotype as previously described (5). All pups were weaned at 21 d of age. Throughout the experiments, mice were housed individually and exposed to a 12-h light/12-h dark cycle. Food and water were given ad libitum. All of the animal experiments were approved by the Purdue Animal Care and Use Committee.
Confirmation of reduced tissue expression of VDR in heterozygous mice
Examination of VDR mRNA Levels in duodenum
To confirm the consequence of deleting VDR alleles on tissue VDR gene expression, we examined the VDR mRNA level in the duodenum of WT, HT, and VDR KO mice. RNA was isolated from mucosal scrapings of the first 2 cm of duodenum from 2-month-old female mice (n = 6 per genotype group) using TriReagent according to the manufacturer's directions (Molecular Research Center Inc., Cincinnati, OH). The isolated RNA was reverse transcribed into cDNA as previously described (29). Real-time PCR was conducted on samples using the My iQ RT-PCR system containing SYBR green (Bio-Rad, Hercules, CA). VDR mRNA levels were normalized to the expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) within the sample and are expressed as relative to the wild-type mouse expression level. PCR conditions for VDR and GAPDH analysis and primers for analysis of GAPDH were previously reported by our group (13). The primers for VDR were: forward, 5′-ATGGAGGCAATGGCAGCCAGCACCTC-3′; reverse, 5′-GAAACCCTTGCAGCCTTCACAGGTCA-3′, and annealing temperature was 61 C. These primers span the 5′ region of the VDR message, and the reverse primer is within exon 2, the section deleted from the Tokyo VDR knockout mouse. As a result, no message is detected from the knockout allele.
Examination of VDR protein levels in kidney
Two-month-old female mice of each genotype (n = 3 per genotype) were killed, and their kidneys were immediately homogenized in buffer of 10 mM Tris-HCl, 500 mM KCl, 1.5 mM EDTA, 10 mM sodium molybdate, and 5 mM dithiothreitol containing 200 μg/ml soybean trypsin inhibitor, 4 μg/ml aprotinin, 4 μg/ml leupeptin, 0.1 mM phenylmethylsulfonyl fluoride, and 0.5 tablet of complete protease inhibitor (Roche Diagnostics, Mannheim, Germany). Samples were centrifuged at 100,000 × g for 1 h at 4 C,and the protein content was determined using the Bradford dye binding assay (Bio-Rad). Forty micrograms of protein were added to each lane of a 16% Tris-glycine gel (Bio-Rad). After electrophoresis and transfer to polyvinyl difluoride membranes, VDR levels were determined by Western blot analysis as previously described (12) with the following modifications: 5% milk in PBS-Tween 20 was used as the blocking buffer, the primary antibody was a 1:4000 dilution of an affinity-purified antibody raised in goats against the N-terminal 21 amino acids of the rat VDR (Ab 4707, a gift from Dr. Nicholas Koszewski, University of Kentucky, Lexington, KY), and the secondary antibody was a 1:5000 dilution of a horseradish peroxidase (HRP)-linked rabbit, antigoat IgG. Specific binding was detected using the ECL Plus kit (Amersham, Piscataway, NJ) and recorded using Quantity One software (Bio-Rad).
Time course and dose response to 1,25(OH)2 D injection
HT and WT mice were raised on a chow diet until 90 d of age. At this point they were fed a low-calcium (0.02%), 0.8% strontium diet (D10373A; Research Diet, New Brunswick, NJ) for 7 d to inhibit endogenous synthesis of 1,25 (OH)2 D3 (30, 31). On the eighth day of strontium feeding, experiments on the time course and dose response to 1,25(OH)2 D3 were conducted. In the time-course study, mice were injected with a single dose of 1,25(OH)2 D3 (Biomol, Plymouth Meeting, PA) [ip, 200 ng per 100 g body weight (BW) in 0.1 ml 10% ethanol and 90% propylene glycol], and killed at 1, 3, 6, and 16 h after the injection (n = 3 males and 3 females per genotype at each time point). Control animals received an ip injection of 0.1 ml of vehicle and were killed at 16 h after the injection. In the dose-response study, mice were injected with either 0.1 ml of vehicle or one dose of 1,25 (OH)2 D3 (ip, 25, 50, 100 ng per 100 g BW, n = 5 males and 5 females of each genotype per dose). Mice were killed 6 h after the injection [a time determined to be optimal for TRPV6 mRNA induction in other experiments (13)]. In both studies the right kidney and a duodenal scraping were harvested, total RNA was isolated, and gene expression was analyzed via real-time RT-PCR. TRPV6, calbindin D9k, 25 hydroxyvitamin D-24 hydroxylase (CYP24), and GAPDH mRNA were examined in the duodenal scraping, whereas TRPV5 and CYP24 mRNA were examined in the kidney using primers and conditions reported previously (5, 13). The primers for CYP24 were: forward, 5′-GCTACGCAAAGGAACAGTCTTAAC-3′ and reverse, 5′-TTGGATTATCCAGCAAAGAGCC-3′, whereas the annealing temperature used was 54 C.
Dietary manipulation of serum 1,25(OH)2D3
HT and WT mice were raised on a commercial chow diet until 90 d of age and then randomly assigned to one of three AIN-76A-based diets (32) containing a normal [0.5% calcium (Ca), 0.4% phosphorus (P)], low (0.02% Ca and 0.35% P), or high calcium content (2.0% Ca, 20% lactose, 1.25% P) (Research Diets) (n = 5 males and 5 females per genotype per diet group) (13). After 7 d on the experimental diets, mice were killed and a duodenal scraping was harvested for analysis of calbindin D9k, TRPV6, and GAPDH mRNA levels via RT-PCR as we have previously described (13) and for calbindin D9k protein analysis via ELISA as described below (n = 5 male mice per genotype per diet). Renal 25-hydroxyvitamin D 1α-hydroxylase (CYP27B1) mRNA levels were analyzed via RT-PCR using primer sets and conditions previously reported by Healy et al. (33). A second set of mice (n = 8-11 per genotype per diet group balanced for gender) was raised under the same protocol and intestinal calcium absorption from a test dose (2 mmol/liter Ca) was examined using in situ ligated loops as previously described (5). The low-calcium load is used to measure the transcellular portion of calcium absorption (34). For both sets of mice, blood was collected into heparinized tubes by cardiac puncture at the time the animals were killed, and plasma was prepared for later analysis. Plasma was analyzed for 1,25(OH)2 D levels using a RIA, and PTH was determined using an immunoenzyme-metric assay (IDS Inc., Fountain Hills, AZ).
Calbindin D9k protein analysis
The duodenal scraping was homogenized and cytosolic extracts prepared as previously described (5). Calbindin D9k protein was examined using a competitive ELISA. Microtiter (96-well) plates were coated with 35 ng/ml of bovine calbindin D9k (Sigma) in borate-buffered saline (BBS) buffer [0.1 m boric acid, 25 mM sodium borate, 150 mM NaCl (pH 8.2)] and incubated at 4 C overnight. The next day, 3% gelatin/BBS was added to each well and incubated for1hat37C. After washing with PBS-Tween (0.05% Tween 20 in PBS, five times), standards (0-100 ng/ml), and samples in 1% gelatin/BBS were added to each well along with a rabbit, antirat calbindin D9k antibody (1:1000 dilution; Swant, Bellinzona, Switzerland). Samples were incubated for 1 h at 37 C. After washes with PBS-Tween buffer (five times), HRP-linked donkey antirabbit IgG was added (1:3000 dilution; Amersham Life Sciences, Arlington Heights, IL) and incubated for 1 h at 37 C. After washing with PBS-Tween buffer (five times), HRP detection was conducted using the HRP substrate kit following the manufacturer's instruction (Bio-Rad). Calbindin D9k levels within the samples were determined from the standard curve. The intra-assay coefficient of variation is 16% and the interassay coefficient of variation is 18% for this assay.
Statistical analysis
Data were analyzed using the SAS statistical program by the general linear models procedure (version 8.0; SAS Institute, Cary, NC). When the plots of predicted values vs. residuals demonstrated that the data were not normally distributed, a log transformation was conducted before statistical analysis. Comparisons of multiple group means were done using Fisher's protected least significant differences (LSD). Regression analysis was conducted using the general linear models and contrast procedures.
We have previously shown that gender influences vitamin D-regulated intestinal calcium absorption and gene expression in mice (35). To account for this, we took several precautions: 1) the number of male and female mice was balanced in all of our experiments; 2) studies were analyzed by ANOVA to assess main effects of treatment, VDR level, and gender and also to identify interactions; whereas gender effects on gene induction and calcium absorption were observed, no significant gender by treatment interactions were found; and 3) to minimize the impact of the independent gender effect on the analysis and increase the power to detect genotype by treatment interactions, gender was used as a covariate. For all analyses, differences between group means or regression parameters (intercept, slope) were considered significant at the P < 0.05 level. Data are expressed as mean ± SEM.
Results
Tissue VDR expression
Yoshizawa et al. (26) previously showed that the intestine of the mouse heterozygous for the VDR null allele has reduced VDR protein levels. We confirmed the observation of reduced tissue VDR expression in two ways. First we found that VDR mRNA was absent in the Tokyo VDR KO mouse and was 50% lower in the duodenum of HT mice (Fig. 1A). In addition, using kidney as a representative tissue, we confirmed that VDR protein was absent in the VDR KO mice and that expression of the VDR protein was reduced by approximately 50% in HT mice, compared with WT mice (Fig. 1B).
FIG. 1.
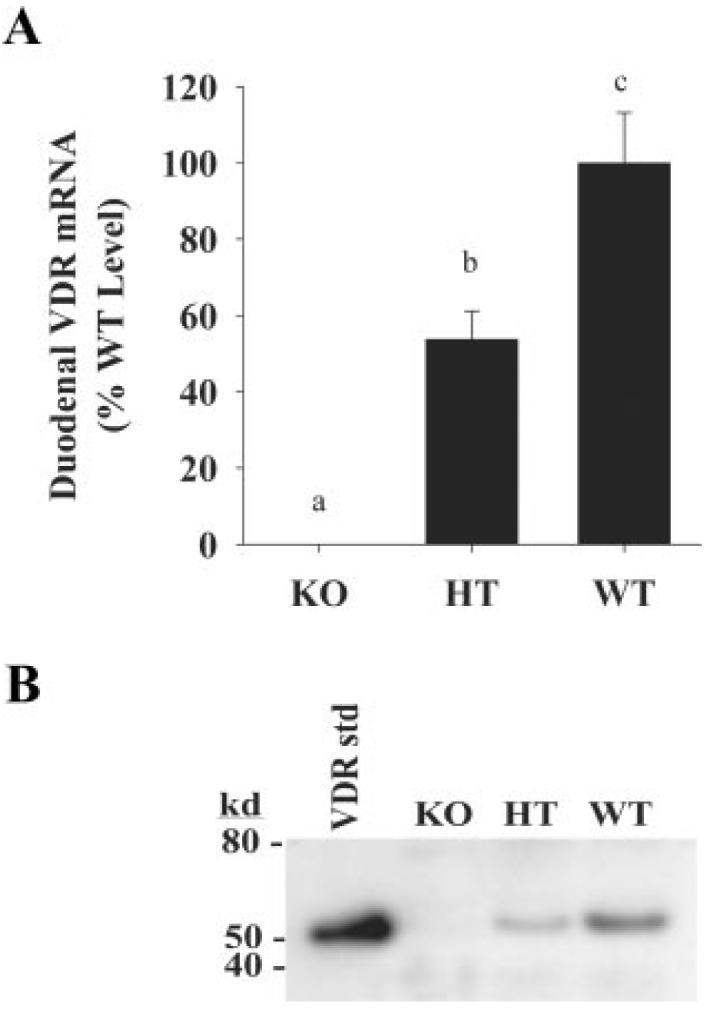
Effect of genotype on tissue VDR expression. Duodenal VDR mRNA (A) or renal VDR protein levels (B) from WT, VDR KO, and HT mice. For the VDR, mRNA analysis bars represent the mean ± SEM (n = 6 per group) and bars with different letter superscripts are significantly different from one another (Fisher's protected LSD, P < 0.05). For the VDR protein analysis, data are representative and the VDR standard is a recombinant human protein.
Gene induction by 1,25(OH)2 D3 injection
To study the molecular role of VDR level as a ligandinducible transcription factor, we examined the acute response of duodenal TRPV6, CYP24, and calbindin D9k mRNA and renal TRPV5 and CYP24 mRNA to a single dose of 1,25(OH)2 D3. In a time-course experiment, plasma 1,25(OH)2 D3 levels were markedly increased (>2000 pg/ml) at 1 h but returned to basal levels by 16 h after injection (116.7 pg/ml).
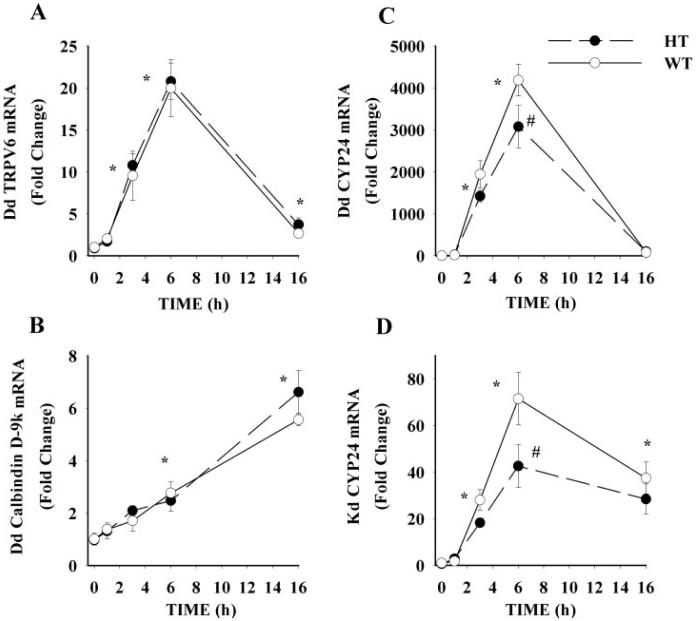
Similar to our previous reports (5, 13, 35), expression of duodenal TRPV6, calbindin D9k, and CYP24 mRNA and renal CYP24 mRNA were significantly increased by 1,25(OH)2 D3 treatment (Fig. 2); TRPV6 and CYP24 mRNA levels were maximally induced by 6 h, whereas calbindin D9k mRNA slowly accumulated along the time course (maximal induction at 16 h). However, we found that there was no significant difference in the induced expression of either duodenal TRPV6 or calbindin D9k mRNA between WT and HT mice (P = 0.48 and P = 0.48, respectively, Fig. 2, A and B). Similarly, renal TRPV5 mRNA levels were maximally induced by 6 h after treatment, but there were no differences in expression between WT and HT mice (data not shown).
FIG. 2.
Effect of VDR level on the induction of duodenal (Dd) and renal (Kd) gene expression. Ninety-day-old WT and HT mice were injected with a single dose of 1,25(OH)2 D3 (200 ng/100 g BW) or vehicle (control). Animals were killed at indicated time and tissues were harvested for mRNA analysis by real-time PCR. Values for specific targets were normalized to the expression of the constitutively expressed gene GAPDH: A, Dd TRPV6; B, Dd calbindin D9k; C, Dd CYP24; and D, Kd CYP24 mRNA. Gender was balanced across genotype and diet groups and used as a covariate to correct for the higher efficiency of calcium absorption previously seen in female mice (35). Values are expressed as fold change relative to the 0 h time point. Symbols represent the mean ± SEM (n = 6). *, Value different from 0 h for both groups; #, HT value significantly lower than WT value (Fisher's protected LSD, P < 0.05).
In the dose-response study, duodenal TRPV6 and calbindin D9k mRNA levels were maximally induced by the 50 ng 1,25(OH)2 D3 per 100 g BW dose, but there was no significant difference between the induction seen in HT and WT mice at any dose tested (data not shown).
In contrast, the induction of duodenal and renal CYP24 mRNA was significantly blunted in HT mice in both the time-course and dose-response studies. HT mice had lower duodenal CYP24 mRNA levels at 3 and 6 h (nonsignificantly lower at 3 h, P = 0.115, and significantly lower at 6 h, P < 0.001) and lower renal CYP24 mRNA levels at 6 h (P < 0.001) after injection in the time-course study (Fig. 2, C and D). In the dose-response study, HT mice injected with either 50 or 100 ng 1,25(OH)2 D3 per 100 g BW had significantly lower induction of duodenal (41 and 30% lower, respectively) and renal (49 and 33% lower, respectively) CYP24 mRNA, compared with WT mice (data not shown).
Diet-induced changes in calcium metabolism and gene expression
As we have previously reported (13, 35), adapting mice to diets with decreasing Ca content significantly increased plasma 1,25(OH)2 D3 levels (Fig. 3C), and this was accompanied by significant elevations in plasma PTH (Fig. 3A) and renal CYP27B1 mRNA levels (Fig. 3B). For each of these parameters, dietary calcium restriction led to a significantly higher level in HT compared with WT mice (88, 55, and 37% higher for PTH, CYP27B1 mRNA, and 1,25(OH)2 D3, respectively). Small but significant differences in plasma PTH and 1,25(OH)2 D3 levels were also seen between HT and WT mice on the 0.5% calcium diet. This reveals a hormonal phenotype of the HT mouse was masked in previous studies using typical high-calcium chow-style diets.
FIG. 3.
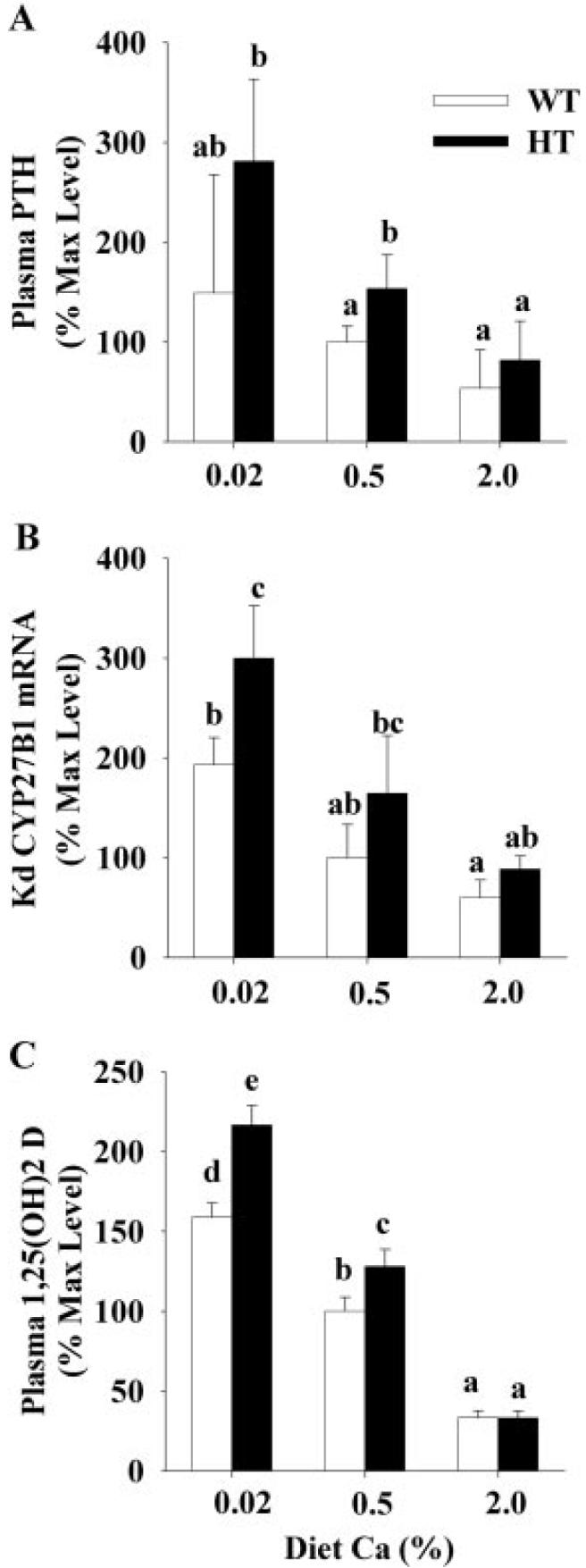
Effect of VDR level on the hormonal adaptation to variations in dietary calcium intake. Ninety-day-old mice were adapted to diets containing different calcium contents (0.5% Ca, 0.02% Ca, or 2.0% Ca) for a 7-d period before the experiment. Plasma was prepared from blood and analyzed for PTH (A) and 1,25(OH)2 D3 (C) using commercial assays. RNA was isolated from kidneys and analyzed for renal (Kd) CYP27B1 mRNA levels by real time RT-PCR and normalized for the expression of GAPDH (B). Gender was balanced across genotype and diet groups and used as a covariate to correct for previously reported gender differences (35). Values are expressed relative to the WT mice fed the 0.5% Ca diet. Bars represent the mean ± SEM (n = 8-11 for PTH and 1,25(OH)2 D3, n = 6 for CYP27B1). Within a panel, bars with different letter superscripts are significantly different from one another (Fisher's protected LSD, P < 0.05).
When calcium absorption was compared by diet group, there was no significant difference between the values for HT and WT mice (data not shown). However, as seen in Fig. 4, the calcium absorption values in HT mice were attained with elevated plasma 1,25(OH)2 D3 levels indicating intestinal resistance to the hormone. To better assess the effect of VDR levels on duodenal calcium absorption, we took advantage of the expanded range of plasma 1,25(OH)2 D3 levels resulting from our dietary calcium manipulation and conducted a regression analysis to determine whether VDR level significantly influenced the response of calcium absorption, TRPV6 mRNA, calbindin D9k mRNA, or calbindin D9k protein to 1,25(OH)2 D3.
FIG. 4.
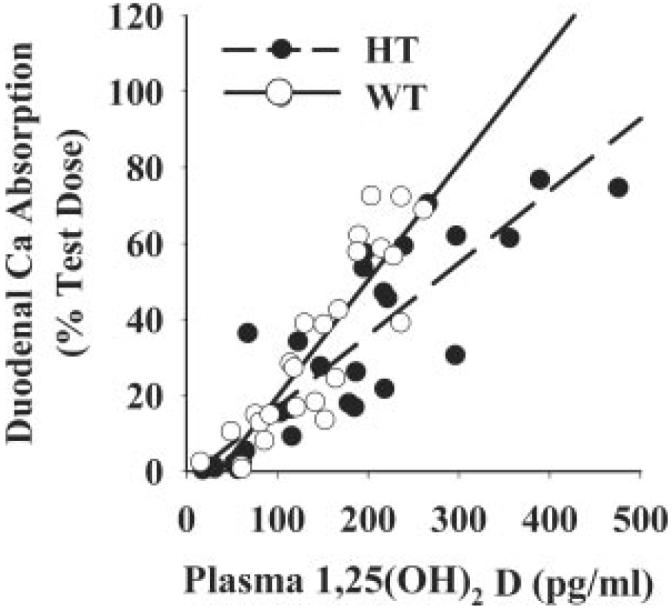
Effect of VDR level on the relationship between intestinal calcium absorption and plasma 1,25(OH)2 D3 levels. Ninety-day-old mice were fed diets containing different levels of calcium (0.5% Ca, 0.02% Ca, or 2.0% Ca) for a 7-d period before the experiment to modulate plasma levels of 1,25(OH)2 D3 in the mice (n = 8-11/diet group). Gender was balanced across genotype and diet groups and used as a covariate to correct for the higher efficiency of calcium absorption previously seen in female mice (35). Calcium absorption values and plasma 1,25(OH)2 D3 data for individual mice was plotted and regression analysis was conducted. Covariate corrected regression lines are presented by solid (WT) or dashed (HT) lines. WT calcium absorption = 0.31 * 1,25(OH)2 D2 - 11.2 (r2 = 0.85); HT calcium absorption = 0.19 * 1,25(OH)2 D2 - 2.3 (r2 = 0.77, WT vs. HT slope, P < 0.01).
For calcium absorption, the slope of the line defining the WT response to increasing levels of plasma 1,25(OH)2 D3 [percent Ca absorption = 0.31 (plasma 1,25(OH)2 D3) - 11.2, r2 = 0.85] was significantly steeper than that of HT mice [percent Ca absorption = 0.19 (plasma 1,25(OH)2 D3) - 2.3, r2 = 0.77; P < 0.001; Fig. 4]. The impact of VDR level was also seen when male and female mice were examined separately, with the exception that the overall relationship between calcium absorption and plasma 1,25(OH)2 D3 levels was higher in female mice [data not shown (35)]. There was also a trend toward a significant difference in the relationship between plasma 1,25(OH)2 D3 and duodenal calbindin D9k protein level in WT [calbindin D9k = 0.046 (plasma 1,25(OH)2 D3) + 5.1, r2 = 0.76) and HT mice (calbindin D9k = 0.025 (plasma 1,25(OH)2 D3) + 5.8; r2 = 0.51, Fig. 5, P = 0.054 for the lower slope in HT mice]. In contrast, there were no significant differences between WT and HT mice for the relationship between plasma 1,25(OH)2 D3 levels and either TRPV6 or calbindin D9k mRNA (data not shown).
FIG. 5.
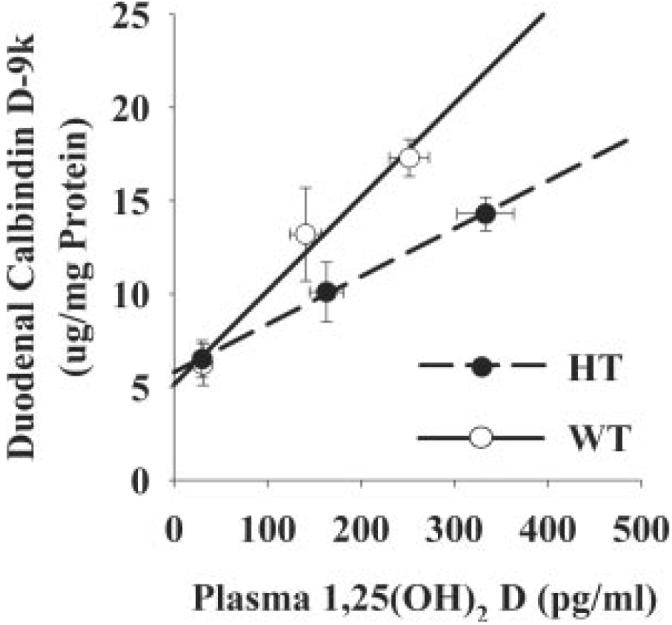
Effect of VDR level on the relationship between calbindin D9k protein levels and plasma 1,25(OH)2 D3 levels. Ninety-day-old mice were fed diets containing different levels of calcium (0.5% Ca, 0.02% Ca, or 2.0% Ca) for a 7-d period before the experiment to modulate plasma levels of 1,25(OH)2 D3 in the mice (n = 5 males/diet group). Calbindin D9k values and plasma 1,25(OH)2 D3 data were plotted by individual mouse or, as shown in the figure, by the mean value for each diet and genotype group, and regression analysis was conducted. Regression lines are presented by solid (WT) or dashed (HT) lines. WT calbindin D9k = 0.046 * 1,25(OH)2 D3 + 5.1 (r2 = 0.76 for individual data points, r2 = 0.98 for means); HT calbindin D9k = 0.026 * 1,25(OH)2 D3 + 5.8 (r2 = 0.51 individual/r2 = 0.99 means, WT vs. HT slope, P = 0.054).
Discussion
Our data reveal that, compared with WT mice, HT mice require higher circulating levels of 1,25(OH)2 D3 to normalize intestinal calcium absorption in response to calcium restriction. This vitamin D resistance resembles the age-associated intestinal resistance to circulating 1,25(OH)2 D3 that we previously reported in rats (18) and that others reported in humans (19, 20). Several groups hypothesized that reduced tissue VDR content accounts for the reduced intestinal calcium absorption that is associated with aging (15) or estrogen deficiency (23). In addition, others have identified high intestinal VDR levels as the mechanism accounting for elevated calcium absorption in the genetic hypercalciuric rat (36). This is consistent with our earlier observations in VDR overexpressing Caco-2 cells (25). However, our studies in HT and WT mice are the first to formally test the hypothesis that reduced VDR level causes intestinal 1,25(OH)2 D3 resistance and calcium malabsorption in vivo.
We initially hypothesized that low VDR content would cause intestinal resistance to 1,25(OH)2 D3 by uniformly reducing vitamin D-induced expression of genes whose protein products are involved in the control of intestinal calcium absorption (13), e.g. TRPV6, calbindin D9k, and CYP24 [the 24 hydroxylase that catalyzes the first step in the inactivation of 1,25(OH)2 D3]. However, only the vitamin D-induced level of CYP24 mRNA (in both duodenum and kidney) was sensitive to VDR content. The CYP24 gene is strongly regulated at the transcriptional level through coordinate VDR-retinoid X receptor interactions at proximal and distal vitamin D response elements (37, 38). Our observation of VDR level sensitivity for vitamin D-regulated CYP24 gene expression is consistent with earlier data from our laboratory in Caco-2 cells (25) and with data on VDR level and CYP24 gene expression in the osteoblast-like cell lines MG-63 and UMR 106 (39). In addition, the effect of VDR level on the CYP24 promoter is similar to the reduced induction of osteocalcin mRNA by 1,25(OH)2 D3 that Martinez et al. found in hip-derived human osteoblast-like cells from donors older than 50 yr whose cells have reduced VDR mRNA levels (40).
In contrast, neither vitamin D-regulated TRPV6 nor calbindin D9k mRNA accumulation was sensitive to lower VDR level. Our previous studies suggest that vitamin D-mediated regulation of intestinal calcium absorption is dependent on induction of these genes (5, 12, 13). However, the mechanisms by which 1,25(OH)2 D3 controls their expression is not clear. Recently Meyer et al. (41) used a chromatin immuno-precipitation scanning method to identify three functional vitamin D response elements (VDREs) in the distal promoter region of the TRPV6 gene promoter (i.e. at -4.3 kb, -2.1 kb, and -1.2 kb). Thus, there are many differences in the TRPV6 and CYP24 gene promoters that could account for differential sensitivity to VDR level, e.g. the number of VDREs in each promoter, location or the local DNA environment of each VDRE in a promoter, or affinity of each VDRE for VDR. Fowler et al. (42) recently showed that high estrogen receptor-α levels in mammary epithelial cells expands the set of estrogen responsive genes and permits ligand-independent and steroid receptor coactivator-3-independent regulation of transcription. It is not clear whether similar mechanisms mediate the impact of VDR level on gene expression in its target tissues. Further analysis of the TRPV6 promoter and other vitamin D-responsive promoters will be necessary to understand how VDR level influences 1,25(OH)2 D3-regulated gene expression in some, but not other, promoters.
Whereas low VDR level did not influence vitamin D-regulated calbindin D9k mRNA levels, it did blunt the relationship between plasma 1,25(OH)2 D3 and duodenal calbindin D9k protein levels. This suggests that the VDR-mediated regulation of calbindin D9k level is not dependent simply on a classical transcriptional response. Consistent with this, we previously reported that the putative VDREs within the human calbindin D9k promoter are inactive in the Caco-2 clone, TC7 (43), and Colnot et al. (44) have shown that vitamin D regulation of the rat calbindin D9k gene promoter in transgenic mice is not influenced by mutation of a putative VDRE at -489 to -475 bp of the promoter (45). This suggests that the impact of vitamin D treatment on calbindin D9k expression is primarily posttranscriptional. This interpretation is supported by studies from Dupret et al. (46, 47), who previously showed that 1,25(OH)2 D3-induced calbindin D9k mRNA accumulation was not blocked by the transcription inhibitor actinomycin D and that transcriptional activation of the calbindin D9k promoter by 1,25(OH)2 D3 in rat duodenum is modest and transient (elevated 3-fold only at 1 and 3 h after injection), even though calbindin D9k mRNA levels continue to accumulate for 16 h after the injected (10-fold increase). In this light, our data suggest that the vitamin D-dependent, translational control of calbindin D9k protein production, but not posttranscriptional control of calbindin D9k mRNA level, is sensitive to VDR level. Translational control of calbindin D9k protein production was previously demonstrated by Armbrecht et al. (48), who showed that the level of duodenal calbindin D9k protein, but not mRNA, was significantly reduced in 12-month-old rats, compared with 2-month-old rats after a single injection of 1,25(OH)2 D3. Although Armbrecht et al. did not measure VDR levels in their study, their findings support the hypothesis that the lower VDR levels thought to exist in older rats (15) are associated with reduced translation of the calbindin D9k mRNA into protein. Further studies are necessary to understand the mechanism of 1,25(OH)2 D3-regulated, VDR level-sensitive, translational control of calbindin D9k protein production.
In summary, we have demonstrated that reduced intestinal VDR level causes vitamin D resistance and impairs transcellular intestinal calcium absorption in mice. However, the blunted intestinal response to 1,25(OH)2 D3 in mice with reduced VDR is not uniform across the molecular responses to hormone, i.e. there is differential sensitivity of promoters to VDR level. Whereas the mechanism for blunted vitamin D regulated intestinal calcium absorption in HT with low VDR content is not clear, our data suggest that this resistance results from VDR-dependent, translational influences of 1,25(OH)2 D3 on calbindin D9k protein production.
Acknowledgments
The authors thank Ms. Christy Gliniak, Dr. Robin Hopkins, Ms. Candace Langdoc, and Mr. Zhentao Zhang for their excellent technical assistance on this project. We also thank Dr. Shigeaki Kato for providing us with breeding pairs of VDR heterozygous mice.
This work was supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Award DK054111 (to J.C.F.).
Glossary
Abbreviations
- BBS
Borate-buffered saline
- BW
body weight
- CYP24
25-hydroxyvitamin D 24-hydroxylase
- CYP27B1
25-hydroxyvitamin D, 1α-hydroxylase
- GAPDH
glyceraldehydes phosphate dehydrogenase
- HRP
horseradish peroxidase
- HT
VDR null heterozygous
- LSD
least significant differences
- 1,25(OH)2 D
1,25 dihydroxyvitamin D
- 1,25(OH)2 D3
1,25 dihydroxyvitamin D3
- P
phosphorus
- PBS-Tween
Tween 20 in PBS
- TRPV6
transient receptor potential channel, vallinoid subfamily member 6
- VDR
vitamin D receptor
- VDRE
vitamin D response element
- VDR KO
VDR knockout
- WT
wild type
Footnotes
Disclosure Summary: J.C.F. has received lecture fees from Wyeth Consumer Healthcare and Eli Lilly and Co.
References
- 1.Ensrud KE, Duong T, Cauley JA, Heaney RP, Wolf RL, Harris E, Cummings SR. Low fractional calcium absorption increases the risk for hip fracture in women with low calcium intake. Study of Osteoporotic Fractures Research Group. Ann Intern Med. 2000;132:345–353. doi: 10.7326/0003-4819-132-5-200003070-00003. [DOI] [PubMed] [Google Scholar]
- 2.Nordin BE, O'Loughlin PD, Need AG, Horowitz M, Morris HA. Radiocalcium absorption is reduced in postmenopausal women with vertebral and most types of peripheral fractures. Osteoporos Int. 2004;15:27–31. doi: 10.1007/s00198-003-1493-1. [DOI] [PubMed] [Google Scholar]
- 3.Bronner F, Pansu D, Stein WD. An analysis of intestinal calcium transport across the rat intestine. Am J Physiol. 1986;250:G561–G569. doi: 10.1152/ajpgi.1986.250.5.G561. [DOI] [PubMed] [Google Scholar]
- 4.Van Cromphaut SJ, Dewerchin M, Hoenderop JG, Stockmans I, Van Herck E, Kato S, Bindels RJ, Collen D, Carmeliet P, Bouillon R, Carmeliet G. Duodenal calcium absorption in vitamin D receptor-knockout mice: functional and molecular aspects. Proc Natl Acad Sci USA. 2001;98:13324–13329. doi: 10.1073/pnas.231474698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song Y, Kato S, Fleet JC. Vitamin D receptor (VDR) knockout mice reveal VDR-independent regulation of intestinal calcium absorption and ECaC2 and calbindin D9k mRNA. J Nutr. 2003;133:374–380. doi: 10.1093/jn/133.2.374. [DOI] [PubMed] [Google Scholar]
- 6.Peng JB, Chen XZ, Berger UV, Vassilev PM, Tsukaguchi H, Brown EM, Hediger MA. Molecular cloning and characterization of a channel-like transporter mediated intestinal calcium absorption. J Biol Chem. 1999;274:22739–22746. doi: 10.1074/jbc.274.32.22739. [DOI] [PubMed] [Google Scholar]
- 7.Peng JB, Chen XZ, Berger UV, Weremowicz S, Morton CC, Vassilev PM, Brown EM, Hediger MA. Human calcium transport protein CaT1. Biochem Biophys Res Commun. 2000;278:326–332. doi: 10.1006/bbrc.2000.3716. [DOI] [PubMed] [Google Scholar]
- 8.Christakos S, Gill R, Lee S, Li H. Molecular aspects of the calbindins. J Nutr. 1992;122:678–682. doi: 10.1093/jn/122.suppl_3.678. [DOI] [PubMed] [Google Scholar]
- 9.Feher JJ. Facilitated calcium diffusion by intestinal calcium-binding protein. Am J Physiol. 1983;244:303–307. doi: 10.1152/ajpcell.1983.244.3.C303. [DOI] [PubMed] [Google Scholar]
- 10.Wasserman RH, Smith CA, Brindak ME, Detalamoni N, Fullmer CS, Penniston JT, Kumar R. Vitamin-D and mineral deficiencies increase the plasma membrane calcium pump of chicken intestine. Gastroenterology. 1992;102:886–894. doi: 10.1016/0016-5085(92)90174-w. [DOI] [PubMed] [Google Scholar]
- 11.Centeno VA, Diaz de Barboza GE, Marchionatti AM, Alisio AE, Dallorso ME, Nasif R, Tolosa de Talamoni NG. Dietary calcium deficiency increases Ca2+ uptake and Ca2+ extrusion mechanisms in chick enterocytes. Comp Biochem Physiol A Mol Integr Physiol. 2004;139:133–141. doi: 10.1016/j.cbpb.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Fleet JC, Eksir F, Hance KW, Wood RJ. Vitamin D-inducible calcium transport and gene expression in three Caco-2 cell lines. Am J Physiol. 2002;283:G618–G625. doi: 10.1152/ajpgi.00269.2001. [DOI] [PubMed] [Google Scholar]
- 13.Song Y, Peng X, Porta A, Takanaga H, Peng JB, Hediger MA, Fleet JC, Christakos S. Calcium transporter 1 and epithelial calcium channel messenger ribonucleic acid are differentially regulated by 1,25 dihydroxyvitamin D3 in the intestine and kidney of mice. Endocrinology. 2003;144:3885–3894. doi: 10.1210/en.2003-0314. [DOI] [PubMed] [Google Scholar]
- 14.Alevizaki CC, Ikkos DG, Singhelakis P. Progressive decrease of true intestinal calcium absorption with age in normal man. J Nucl Med. 1973;14:760–762. [PubMed] [Google Scholar]
- 15.Horst RL, Goff JP, Reinhardt TA. Advancing age results in reduction of intestinal and bone 1,25 dihydroxyvitamin D receptor. Endocrinology. 1990;126:1053–1057. doi: 10.1210/endo-126-2-1053. [DOI] [PubMed] [Google Scholar]
- 16.Nordin BE, Need AG, Morris HA, O'Loughlin PD, Horowitz M. Effect of age on calcium absorption in postmenopausal women. Am J Clin Nutr. 2004;80:998–1002. doi: 10.1093/ajcn/80.4.998. [DOI] [PubMed] [Google Scholar]
- 17.Coudray C, Feillet-Coudray C, Rambeau M, Tressol JC, Gueux E, Mazur A, Rayssiguier Y. The effect of aging on intestinal absorption and status of calcium, magnesium, zinc, and copper in rats: a stable isotope study. J Trace Elem Med Biol. 2006;20:73–81. doi: 10.1016/j.jtemb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Wood RJ, Fleet JC, Cashman K, Bruns ME, DeLuca HF. Intestinal calcium absorption in the aged rat: evidence of intestinal resistance to 1,25(OH)2 vitamin D. Endocrinology. 1998;139:3843–3848. doi: 10.1210/endo.139.9.6176. [DOI] [PubMed] [Google Scholar]
- 19.Pattanaungkul S, Riggs BL, Yergey AL, Vieira NE, O'Fallon WM, Khosla S. Relationship of intestinal calcium absorption to 1,25-dihydroxyvitamin D [1,25(OH)2D] levels in young versus elderly women: evidence for age-related intestinal resistance to 1,25(OH)2D action. J Clin Endocrinol Metab. 2000;85:4023–4027. doi: 10.1210/jcem.85.11.6938. [DOI] [PubMed] [Google Scholar]
- 20.Scopacasa F, Wishart JM, Horowitz M, Morris HA, Need AG. Relation between calcium absorption and serum calcitriol in normal men: evidence for age-related intestinal resistance to calcitriol. Eur J Clin Nutr. 2004;58:264–269. doi: 10.1038/sj.ejcn.1601777. [DOI] [PubMed] [Google Scholar]
- 21.Gennari C, Agnusdei D, Nardi P, Civitelli R. Estrogen preserves a normal intestinal responsiveness to 1,25-dihydroxyvitamin D3 in oophorectomized women. J Clin Endocrinol Metab. 1990;71:1288–1293. doi: 10.1210/jcem-71-5-1288. [DOI] [PubMed] [Google Scholar]
- 22.Ebeling PR, Sandgren ME, Dimagno EP, Lane AW, DeLuca HF, Riggs BL. Evidence of an age-related decrease in intestinal responsiveness to vitamin-D—relationship between serum 1,25-dihydroxyvitamin-D3 and intestinal vitamin-D receptor concentrations in normal women. J Clin Endocrinol Metab. 1992;75:176–182. doi: 10.1210/jcem.75.1.1320048. [DOI] [PubMed] [Google Scholar]
- 23.Chen C, Noland KA, Kalu DN. Modulation of intestinal vitamin D receptor by ovariectomy, estrogen and growth hormone. Mech Ageing Dev. 1997;99:109–122. doi: 10.1016/s0047-6374(97)00094-8. [DOI] [PubMed] [Google Scholar]
- 24.Liel Y, Shany S, Smirnoff P, Schwartz B. Estrogen increases 1,25-dihydroxyvitamin D receptors expression and bioresponse in the rat duodenal mucosa. Endocrinology. 1999;140:280–285. doi: 10.1210/endo.140.1.6408. [DOI] [PubMed] [Google Scholar]
- 25.Shao A, Wood RJ, Fleet JC. Increased vitamin D receptor level enhances 1,25-dihydroxyvitamin D3-mediated gene expression and calcium transport in Caco-2 cells. J Bone Miner Res. 2001;16:615–624. doi: 10.1359/jbmr.2001.16.4.615. [DOI] [PubMed] [Google Scholar]
- 26.Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, Kawakami T, Arioka K, Sato H, Uchiyama Y, Masushige S, Fukamizu A, Matsumoto T, Kato S. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet. 1997;16:391–396. doi: 10.1038/ng0897-391. [DOI] [PubMed] [Google Scholar]
- 27.Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, Demay MB. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci USA. 1997;94:9831–9835. doi: 10.1073/pnas.94.18.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erben RG, Soegiarto DW, Weber K, Zeitz U, Lieberherr M, Gniadecki R, Moller G, Adamski J, Balling R. Deletion of deoxyribonucleic acid binding domain of the vitamin D receptor abrogates genomic and nongenomic functions of vitamin D. Mol Endocrinol. 2002;16:1524–1537. doi: 10.1210/mend.16.7.0866. [DOI] [PubMed] [Google Scholar]
- 29.Fleet JC, Wood RJ. Specific 1,25(OH)2 D3-mediated regulation of trans-cellular calcium transport in Caco-2 cells. Am J Physiol. 1999;276:G958–G964. doi: 10.1152/ajpgi.1999.276.4.G958. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka Y, DeLuca HF, Omdahl J, Holick MF. Mechanism of action of 1,25-dihydroxycholecalciferol on intestinal calcium transport. Proc Natl Acad Sci USA. 1971;68:1286–1288. doi: 10.1073/pnas.68.6.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armbrecht HJ, Wasserman RH, Bruns MEH. Effect of 1,25 dihydroxyvitamin D3 on intestinal calcium absorption in strontium-fed rats. Arch Biochem Biophys. 1979;192:466–473. doi: 10.1016/0003-9861(79)90116-4. [DOI] [PubMed] [Google Scholar]
- 32.American Institute of Nutrition Second report of the ad hoc committee for experimental animals. J Nutr. 1980;110:1726. [Google Scholar]
- 33.Healy KD, Zella JB, Prahl JM, DeLuca HF. Regulation of the murine renal vitamin D receptor by 1,25-dihydroxyvitamin D3 and calcium. Proc Natl Acad Sci USA. 2003;100:9733–9737. doi: 10.1073/pnas.1633774100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bronner F, Yost JH. Saturable and nonsaturable copper and calcium transport in mouse duodenum. Am J Physiol. 1985;249:108–112. doi: 10.1152/ajpgi.1985.249.1.G108. [DOI] [PubMed] [Google Scholar]
- 35.Song Y, Fleet JC. 1,25 Dihydroxycholecalciferol-mediated calcium absorption and gene expression are higher in female than in male mice. J Nutr. 2004;134:1857–1861. doi: 10.1093/jn/134.8.1857. [DOI] [PubMed] [Google Scholar]
- 36.Karnauskas AJ, van Leeuwen JP, van den Bemd GJ, Kathpalia PP, DeLuca HF, Bushinsky DA, Favus MJ. Mechanism and function of high vitamin Dreceptor levels in genetic hypercalciuric stone-forming rats. J Bone Miner Res. 2005;20:447–454. doi: 10.1359/JBMR.041120. [DOI] [PubMed] [Google Scholar]
- 37.Kerry DM, Dwivedi PP, Hahn CN, Morris HA, Omdahl JL, May BK. Transcriptional synergism between vitamin D-responsive elements in the rat 25-hydroxyvitamin D3 24-hydroxylase (CYP24) promoter. J Biol Chem. 1996;271:29715–29721. doi: 10.1074/jbc.271.47.29715. [DOI] [PubMed] [Google Scholar]
- 38.Vaisanen S, Dunlop TW, Sinkkonen L, Frank C, Carlberg C. Spatio-temporal activation of chromatin on the human CYP24 gene promoter in the presence of 1α,25-dihydroxyvitamin D3. J Mol Biol. 2005;350:65–77. doi: 10.1016/j.jmb.2005.04.057. [DOI] [PubMed] [Google Scholar]
- 39.Staal A, van den Bemd GJCM, Birkenhager JC, Pols HAP, van Leeuwen JPTM. Consequences of vitamin D receptor regulation for the 1,25 dihydroxyvitamin D3-induced 24-hydroxylase activity in osteoblast-like cells: initiation of the C24-oxidation pathway. Bone. 1997;20:237–243. doi: 10.1016/s8756-3282(96)00371-7. [DOI] [PubMed] [Google Scholar]
- 40.Martinez P, Moreno I, De Miguel F, Vila V, Esbrit P, Martinez ME. Changes in osteocalcin response to 1,25-dihydroxyvitamin D3 stimulation and basal vitamin D receptor expression in human osteoblastic cells according to donor age and skeletal origin. Bone. 2001;29:35–41. doi: 10.1016/s8756-3282(01)00479-3. [DOI] [PubMed] [Google Scholar]
- 41.Meyer MB, Watanuki M, Kim S, Shevde NK, Pike JW. The human transient receptor potential vanilloid type 6 distal promoter contains multiple vitamin D receptor binding sites that mediate activation by 1,25-dihydroxyvitamin D3 in intestinal cells. Mol Endocrinol. 2006;20:1447–1461. doi: 10.1210/me.2006-0031. [DOI] [PubMed] [Google Scholar]
- 42.Fowler AM, Solodin NM, Valley CC, Alarid ET. Altered target gene regulation controlled by estrogen receptor-α concentration. Mol Endocrinol. 2006;20:291–301. doi: 10.1210/me.2005-0288. [DOI] [PubMed] [Google Scholar]
- 43.Wang L, Klopot A, Freund JN, Dowling LN, Krasinski SD, Fleet JC. Control of differentiation-induced calbindin-D9k gene expression in Caco-2 cells by Cdx-2 and HNF-1α. Am J Physiol. 2004;287:G943–G953. doi: 10.1152/ajpgi.00121.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colnot S, Ovejero C, Romagnolo B, Porteu A, Lacourte P, Thomasset M, Perret C. Transgenic analysis of the response of the rat calbindin-D9k gene to vitamin D. Endocrinology. 2000;141:2301–2308. doi: 10.1210/endo.141.7.7557. [DOI] [PubMed] [Google Scholar]
- 45.Darwish HM, DeLuca HF. Identification of a 1,25-dihydroxyvitamin D3-response element in the 5′-flanking region of the rat calbindin D-9k gene. Proc Natl Acad Sci USA. 1992;89:603–607. doi: 10.1073/pnas.89.2.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dupret JM, Brun P, Thomasset M. In vivo effects of transcriptional and translational inhibitors on duodenal vitamin D-dependent calcium-binding protein messenger ribonucleic acid stimulation by 1,25-dihydroxycholecalciferol. Endocrinology. 1986;119:2476–2483. doi: 10.1210/endo-119-6-2476. [DOI] [PubMed] [Google Scholar]
- 47.Dupret JM, Brun P, Perret C, Lomri N, Thomasset M, Cuisinier-Gleizes P. Transcriptional and posttranscriptional regulation of vitamin D-dependent calcium-binding protein gene expression in the rat duodenum by 1,25-dihydroxycholecalciferol. J Biol Chem. 1987;262:16553–16557. [PubMed] [Google Scholar]
- 48.Armbrecht HJ, Boltz MA, Christakos S, Bruns MEH. Capacity of 1,25-dihydroxyvitamin D to stimulate expression of calbindin D changes with age in the rat. Arch Biochem Biophys. 1998;352:159–164. doi: 10.1006/abbi.1998.0594. [DOI] [PubMed] [Google Scholar]