Summary
It is established that the majority but not all of the seizure-induced cell death is associated with status epilepticus while spontaneous recurrent seizures associated with epilepsy do not cause neuronal death. Extracellular effects and compensatory changes in brain physiology complicate assessment of neuronal death in vivo as the result of seizures. In this study we utilized a well-characterized in vitro hippocampal neuronal culture model of both continuous high-frequency epileptiform discharges (status epilepticus) and spontaneous recurrent epileptiform discharges (acquired epilepsy) to investigate the direct effects of continuous and episodic electrographic epileptiform discharges on cell death in a carefully controlled extracellular environment. The results from this study indicate that continuous high-frequency epileptiform discharges can cause neuronal death in a time-dependent manner. Episodic epileptiform seizure activity occurring for the life of the neurons in culture was not associated with increased neuronal cell death. Our data confirm observations from clinical and some animal studies that spontaneous recurrent seizures do not initiate cell death. The hippocampal neuronal culture model provides a powerful in vitro tool for carefully evaluating the effects of seizure activity alone on neuronal viability in the absence of various confounding factors and may provide new insights into the development of novel therapeutic agents to prevent neuronal injury during status epilepticus.
Keywords: Low Mg2+ model of status epilepticus, Acquired epilepsy, Neuronal death, Patch clamp electrophysiology, Hippocampal neuronal cultures, Fluorescein diacetate–propidium iodide
Introduction
It has been well established that status epilepticus (SE) is associated with neuronal cell death (Fujikawa, 2005). The duration and continuous nature of the seizure discharges appear to be primarily associated with brain injury (Lowenstein and Alldredge, 1998; Delorenzo, 2006). Neuronal damage produced by prolonged seizures is dependent upon multiple variables including the location of seizure foci, seizure duration, age, and genetic susceptibility along with exacerbating factors such as the underlying etiology of the seizures, hypoxia and ischemia (Fountain, 2000). Pioneering studies by Meldrum et al. (1973) in ventilated baboons provided the first evidence that electrographic seizures alone in the absence of underlying systemic physiological complications could still cause neuronal death. There is strong evidence that SE can cause significant neuronal damage especially in the limbic system in both animals (Fujikawa, 1996; Holmes, 2002) and man (Duncan, 2002; Men et al., 2000; Schreiber et al., 1999). Conversely, it is now widely accepted in animals and man that spontaneous recurrent seizures, occurring in the context of intractable epilepsy, do not cause significant brain injury (Fujikawa, 2005). Evidence is now accumulating especially from the in vivo models of epilepsy, suggesting that neuronal cell death is associated with initial epileptogenic insult and not with later spontaneous seizures (Gorter et al., 2003; Pitkanen et al., 2002). Understanding the effects of epileptiform discharges on neuronal viability is important in developing therapeutic strategies for treating SE and epilepsy.
In vivo studies are complicated by compensatory environmental changes in the brain that are characteristic of living systems. Cellular responses to SE in the living animal are integrated in a complex manner to regulate metabolic homeostasis, even when ventilation is controlled as in the original studies by Meldrum et al. (1973). Thus, evaluating the effects of electrographic epileptiform activity on electrophysiological and metabolic functions is very difficult to access at the single neuron and molecular levels in vivo, since it is not possible to carefully control the neuronal environment during seizure activity. It is therefore important to evaluate the effects of continuous electrographic seizure activity in comparison to spontaneous recurrent seizures in a controlled environment to directly determine the effects of seizure activity on neuronal viability.
This study was initiated to investigate effects of seizure discharges on neuronal viability in a carefully controlled neuronal environment utilizing the well established hippocampal neuronal culture models of continuous high-frequency epileptiform discharges (status epilepticus) and spontaneous recurrent epileptiform discharges (epilepsy) (Blair et al., 2006; Deshpande et al., 2007a,b,c). The low Mg2+ model of continuous high-frequency epileptiform discharges and the spontaneous recurrent epileptiform discharges (SREDs) in hippocampal neuronal cultures have been routinely used to characterize biochemical, electrophysiological and molecular mechanisms underlying SE and epilepsy in an in vitro settings (Churn et al., 2000; Delorenzo et al., 2005; Goodkin et al., 2005; Mangan and Kapur, 2004). The spike frequency and epileptiform discharges manifested in this hippocampal neuronal culture model mimics the electrographic features observed with in vivo SE and epilepsy (Delorenzo et al., 2005; Mangan and Kapur, 2004). While the neuronal cultures do not have true anatomical connections and do not display clinical seizures, the hippocampal neuronal culture models have been used as powerful tools to study epileptiform activity and have the unique advantage of applying various in vitro techniques to study molecular mechanisms underlying SE and acquired epilepsy. These in vitro models also allow for careful control of the neuronal environment. Thus, these models are ideally suited to evaluate the effects of electrographic seizure activity on neuronal viability in a controlled physiological environment.
The results from this study indicate that continuous high-frequency epileptiform discharges that are analogous to SE can cause neuronal cell death in a time-dependent manner, but that spontaneous recurrent epileptiform discharges occurring for the life of the neurons in culture are not associated with increased neuronal cell death. The data confirm the clinical observations that spontaneous recurrent seizures do not initiate cell death and directly demonstrate for the first time that prolonged continuous electrographic seizure activity in a controlled neuronal environment can exclusively initiate neuronal cell death.
Materials and methods
All the reagents including fluorescein diacetate and propidium iodide were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise noted. Sodium pyruvate, minimum essential media containing Earle’s salts, fetal bovine serum and horse serum were obtained from Gibco-BRL (Invitrogen Corp., Carlsbad, CA).
Hippocampal neuronal culture
All animal use procedures were in strict accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and approved by Virginia Commonwealth University’s Institutional Animal Care and Use Committee. Studies were conducted on primary mixed hippocampal neuronal cultures prepared as described previously with slight modifications (Deshpande et al., 2007a; Sombati and DeLorenzo, 1995). In brief, hippocampal cells were obtained from 2-day postnatal Sprague–Dawley rats (Harlan, Frederick, MD) and plated at a density of 2.5 × 104 cells/cm2 onto a glial support layer previously plated onto poly-l-lysine coated (0.05 mg/ml) 35mm grid cell culture dishes (Nunc, Naperville, IL). Cultures were maintained at 37 °C in a 5% CO2/95% air atmosphere and fed twice weekly with neuronal feed containing 0.5mM l-glutamine.
Hippocampal neuronal culture model of continuous high-frequency epileptiform discharges
After 2 weeks, cultures were utilized for experimentation and continuous high-frequency epileptiform discharges were induced as described previously (Blair et al., 2006; Deshpande et al., 2007a,b; Sombati and DeLorenzo, 1995). Maintenance medium was replaced with physiological recording solution (pBRS) with or without MgCl2 containing (in mM): 145 NaCl, 2.5 KCl, 10 HEPES, 2 CaCl2, 10 glucose, and 0.002 glycine, pH 7.3, and osmolarity adjusted to 290 ± 10 mOsm with sucrose. Continuous epileptiform high-frequency bursts were induced by exposing neuronal cultures to pBRS without added MgCl2 (low Mg2+). The continuous high-frequency epileptiform discharges continued until pBRS containing 1mM MgCl2 was added back to the cultures. Unless indicated as low Mg2+ treatment, experimental protocols utilized pBRS containing 1mM MgCl2.
For the perfusion studies, the low Mg2+ solution was continuously perfused over the culture plate employing standard multi-channel gravity feed perfusion system (Warner Instrument Corp., Hamden, CT) at the rate of 2 ml/min. This procedure does not disrupt the neurons, allows for continuous electrophysiological monitoring and provide a complete exchange of the experimental/maintenance media every 1 min. Thus this technique prevents build up of metabolites released during the continuous epileptiform discharges to provide a more controlled environment for the neurons. Both control and low Mg2+ exposed neurons were treated identically. Glutamate was measured by enzymatic fluorometric assay with a CMA 600 Microdialysis Analyzer (Didier et al., 1990; Sun et al., 2001).
Hippocampal neuronal culture model of SREDs
SREDs were induced in the neuronal cultures by exposing them for 3 h to a solution containing no added MgCl2 (low Mg2+) using procedures described previously (Blair et al., 2006; Deshpande et al., 2007c; Sombati and DeLorenzo, 1995). Briefly, after the removal of maintenance media, neurons were gently washed with 3× 1.5 ml of pBRS (±1mM MgCl2) and then allowed to incubate in this solution at 37 °C under 5% CO2/95% air atmosphere. At the end of the 3 h period, low Mg2+ treatment was terminated by gently washing the neuronal cultures with 3× 1.5 ml of minimum essential medium, returned to the maintenance medium and incubated at 37 °C under 5% CO2/95% air atmosphere. Thus, low Mg2+ treatment was carried out with pBRS without added MgCl2, whereas sham controls were treated with pBRS containing 1mM MgCl2. SREDs are noticed in the neuronal cultures after approximately 12–24 h and they last for the life of neurons in culture.
Neuronal death assay
Neuronal death was assessed at various time points after low Mg2+ exposure using fluorescein diacetate (FDA)–propidium iodide (PI) microfluorometry (Didier et al., 1990; Sun et al., 2001). The two-dye method allows for the identification of viable and non-viable neurons in the same neuronal field. Cell injury causes the loss of FDA staining and permits PI to pass through the damaged cell membrane and interact with DNA to emit bright red fluorescence. Morphological assessment of the cultures was also carried out to determine neuronal viability (Churn et al., 1995; Limbrick et al., 1995). Three randomly selected fields were marked and photographed. Both fluorescent and phase bright images were captured. These same fields were examined at various time points during continual exposure to low Mg2+ solution. Neurons labeled with FDA or PI were quantified by means of the Ultraview image analysis software package (Perkin Elmer Life Sciences) and percent neuronal death was calculated as the number of neurons labeled by PI divided by the sum of the number of neurons labeled by PI and those labeled by FDA. Fluorescent images were compared with phase bright images to confirm that only pyramid-shaped neurons were counted. Three randomly selected fields were counted and averaged per culture (approximately 18–25 neurons per field). Counting was done manually.
Whole cell current clamp recordings
Whole cell current clamp recordings were performed using previously established procedures (Deshpande et al., 2007a,b,c; Sombati and DeLorenzo, 1995). Briefly, cell culture dish was mounted on the stage of an inverted microscope (Nikon Diaphot, Tokyo, Japan) continuously perfused with pBRS. Patch electrodes with a resistance of 2–4mΩ were pulled on a Brown-Flaming P-80C electrode puller (Sutter Instruments, Novato, CA), fire-polished and filled with a solution containing (in mM): 140 K+ gluconate, 1.1 EGTA, 1 MgCl2, and 10 Na–HEPES, pH 7.2, osmolarity adjusted to 290 ± 10 mOsm with sucrose. Whole cell recordings were carried out using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA) in a current clamp mode. Data were digitized via Digidata 1322A (Axon Instruments, Foster City, CA) and transferred to VHS tape using a PCM device (Neurocorder, New York, NY) and then played back on a DC-500 Hz chart recorder (Astro-Med Dash II, Warwick, RI).
Data analyses
Data are reported as mean ± S.E.M. Neuronal cell death data were examined using one-way analysis of variance (ANOVA) followed by a post hoc Tukey’s test. Paired t-tests were utilized to compare differences in neuronal death data between the fluorescent techniques and phase contrast microscopy. Linear regression analysis was also conducted to examine the mean values of cell death as affected by seizure duration. Each neuronal culture plate was treated as n=1. Data were analyzed using SigmaStat 2.0 and plotted using SigmaPlot 8.02 (SPSS Inc., Chicago, IL).
Results
Continuous high-frequency epileptiform discharges in hippocampal neuronal cultures
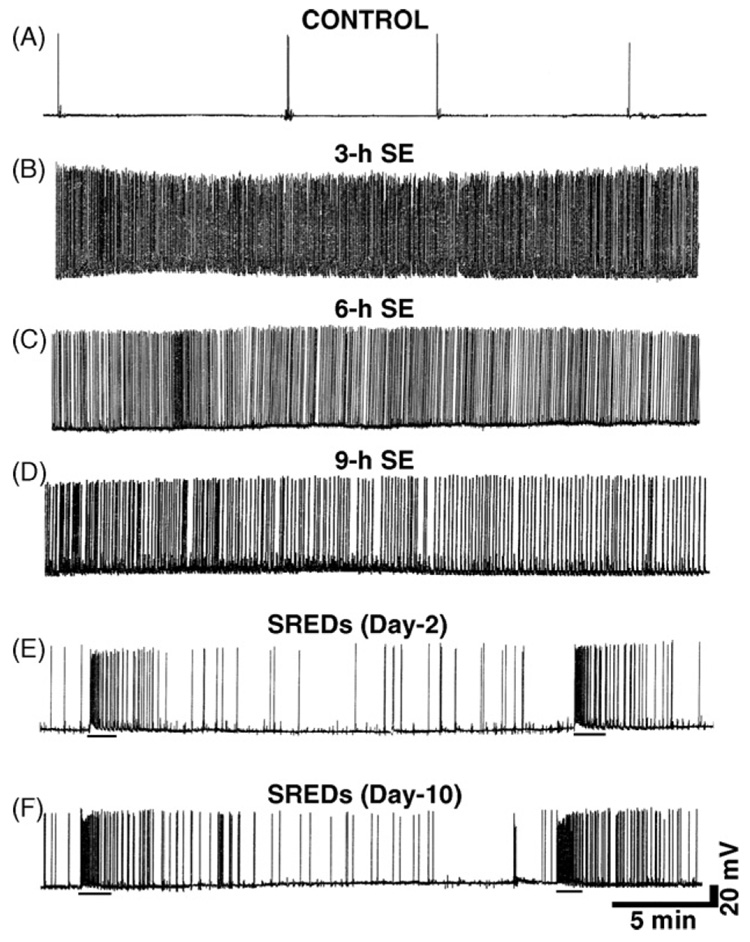
Treatment of hippocampal neurons in culture with low Mg2+-induced large, long duration synaptic potentials with multiple action potentials, evolving into high-frequency burst discharges (Delorenzo et al., 2005; Mangan and Kapur, 2004). Fig. 1B illustrates a 30 min recording from a neuron after 3 h of exposure to low Mg2+. The resultant spike discharges were sustained for the entire low Mg2+ exposure. Expansion of the time scale indicated that spike frequency occurred at 8–15 Hz (data not shown). SE is defined as 30 min or longer of continuous seizure activity with a spike frequency of greater than 3 Hz (Lowenstein and Alldredge, 1998; Delorenzo, 2006). Fig. 1C and D shows spike frequency pattern for continuous high-frequency epileptiform discharges activity recorded at 6 and 9 h of low Mg2+ treatment, respectively. Thus, the high-frequency spiking continued throughout the 9 h period of low Mg2+ treatment. Following re-addition of normal Mg2+ level to the media, these spontaneous discharges were immediately stopped. Recording from a control neuron showed occasional spontaneous action potentials (Fig. 1A).
Figure 1. Continuous high-frequency epileptiform discharges and SREDs in cultured hippocampal neurons.
(A) Representative current clamp recording from a control neuron showing occasional spontaneous action potentials. (B–D) Representative current clamp recordings during low Mg2+ treatment showing induction of tonic high-frequency epileptiform bursts (abbreviated SE in the figure) from neurons subjected to 3, 6 and 9 h of low Mg2+ exposure, respectively. Tonic high-frequency spiking was observed at all the timepoints. While a little slowing of spike activity is sometimes observed at later time points, the spike frequency was still >3 Hz and meets the criteria of SE. (E) A continuous 30 min recording from a representative neuron 2 days following an initial 3 h exposure to low Mg2+ solution. Two SREDs or spontaneous seizure episodes lasting ~60–80 s can be seen in this time frame (denoted by black bars beneath each episode in the trace). (F) A continuous 30 min recording from a representative neuron 10 days following an initial 3 h exposure to low Mg2+ solution. These SREDs at 10 days after initial injury with low Mg2+ are identical to the SREDs seen 2 days after low Mg2+-induced high-frequency spiking (E) and demonstrate that the SREDs phenotype lasts for the life of the neurons in culture in this model.
SREDs in hippocampal neuronal cultures
Whole cell current clamp recordings from neurons in cultures 2 days after a 3 h, low Mg2+ treatment demonstrated SREDs or seizure events. Two SREDs lasting between 1 and 1.5 min are shown in Fig. 1E and were observed in a continuous 30 min recording. These SREDs are characteristic of the epileptic phenotype and were observed for the life of the neurons in culture after the initial insult with low Mg2+. Thus SREDs were also observed even at 10 days after initial SE insult (Fig. 1F). Using multiple recordings from adjacent neurons we have demonstrated previously that these SREDs were synchronous events occurring in populations of neurons (Sombati and DeLorenzo, 1995). SREDs started and stopped spontaneously, had durations between 1 and 2 min and occurred at frequencies of up to 2–4 episodes every hour. We have shown previously that SREDs produced in cultured neurons manifest electrographic properties, neuronal population synchronicity and anticonvulsant sensitivity analogous to those of observed in vivo (Sombati and DeLorenzo, 1995). Age-matched control neurons revealed ‘‘normal’’ baseline activity consisting of intermittent action potentials. SREDs were never observed in control neurons and there were no significant differences in membrane potential and input resistance between control and epileptic neurons (Deshpande et al., 2007c).
Increasing durations of continuous high-frequency epileptiform discharges and its effect on neuronal cell death
We investigated the effects of increasing durations of continuous high-frequency epileptiform discharges on hippocampal neuronal cell death (Fig. 1). Continuous high-frequency epileptiform discharges were induced as described in section ‘Hippocampal neuronal culture model of continuous high-frequency epileptiform discharges’ by removing Mg2+ from the culture medium. In order to standardize the method for cell death assessment, in the first set of experiments low Mg2+ exposure was continued for 0 or 12 h at the end of which neuronal cultures were washed with solution containing normal Mg2+ concentrations (2 mM) and cell death was measured using FDA–PI staining (section ‘Neuronal death assay’).
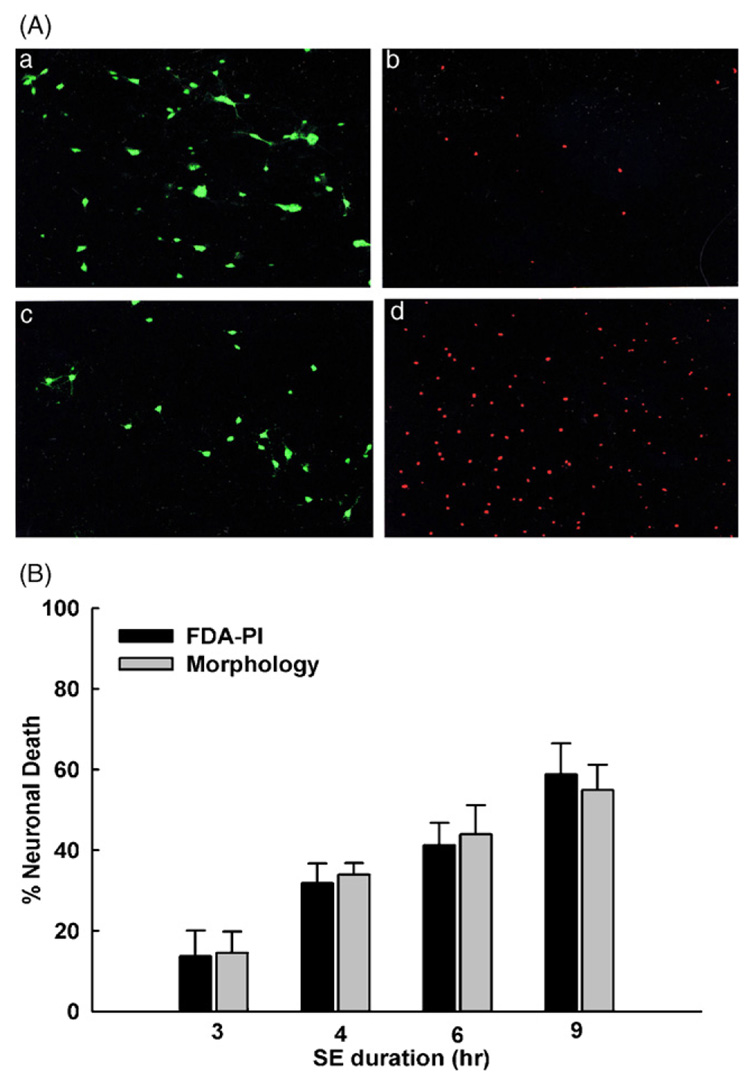
Fig. 2A shows representative fluorescent images from neurons before (0 h) and after (12 h) exposure to low Mg2+-induced continuous high-frequency epileptiform discharges. Intra-vital staining with FDA–PI in cultures that were not exposed to low Mg2+ conditions exhibited a preponderance of cells fluorescing green and few fluorescing red (Fig. 2A, panels a and b). Cultures that were exposed to low Mg2+ conditions displayed a decrease in FDA staining brightness and corresponding increase in PI fluorescence (Fig. 2A, panels c and d).
Figure 2. Neuronal cell death as a function of seizure duration.
(A) Representative images of neurons at 0 and 12 h of low Mg2+-induced high-frequency spiking. Frame a: FDA staining of viable neurons at 0 h (n = 49). Frame b: PI staining of nuclei of non-viable neurons in the same field as (a) at 0 h. Frame c: FDA staining of viable neurons at 12 h of low Mg2+ exposure (n = 32). Frame d: PI staining of non-viable neurons in the same field as (c) at 12 h of low Mg2+ exposure. (B) Comparison of neuronal cell death after 3, 4, 6 and 9 h exposure to low Mg2+ solution (abbreviated SE in the figure). Percentage neuronal cell death is dependent on the duration of exposure to low Mg2+ solution in culture. Gray bars represent cell death assessed by visual examination of neuronal morphology. Black bars indicate cell death examined using FDA–PI intra-vital staining as described in section ‘Materials and Methods’. Differences between the two techniques were not statistically significant (paired t-test). FDA–PI staining was performed 24 h after exposure to low Mg2+ solution for these studies.
To characterize the effects of the duration of continuous high-frequency epileptiform discharges on cell death, neuronal cultures were subjected to varying durations of exposure to low Mg2+ which included 3, 4, 6 and 9 h after which time cell death was quantified using the FDA–PI staining method. To rigorously control for the validity of the FDA–PI staining method, we also quantified neuronal viability by morphological assessment in the same fields of neurons. As shown in Fig. 2B, the results from both the FDA–PI fluorescent dye staining and the neuronal morphology examination techniques were consistent and were not statistically divergent. Paired t-tests were used to compare differences in neuronal death data between the fluorescent techniques and phase contrast microscopy. The FDA–PI technique was accurate and required less time to perform. Thus, the subsequent neuronal viability measurements were made using this technique.
Correlation between duration of continuous high-frequency epileptiform discharges and neuronal cell death
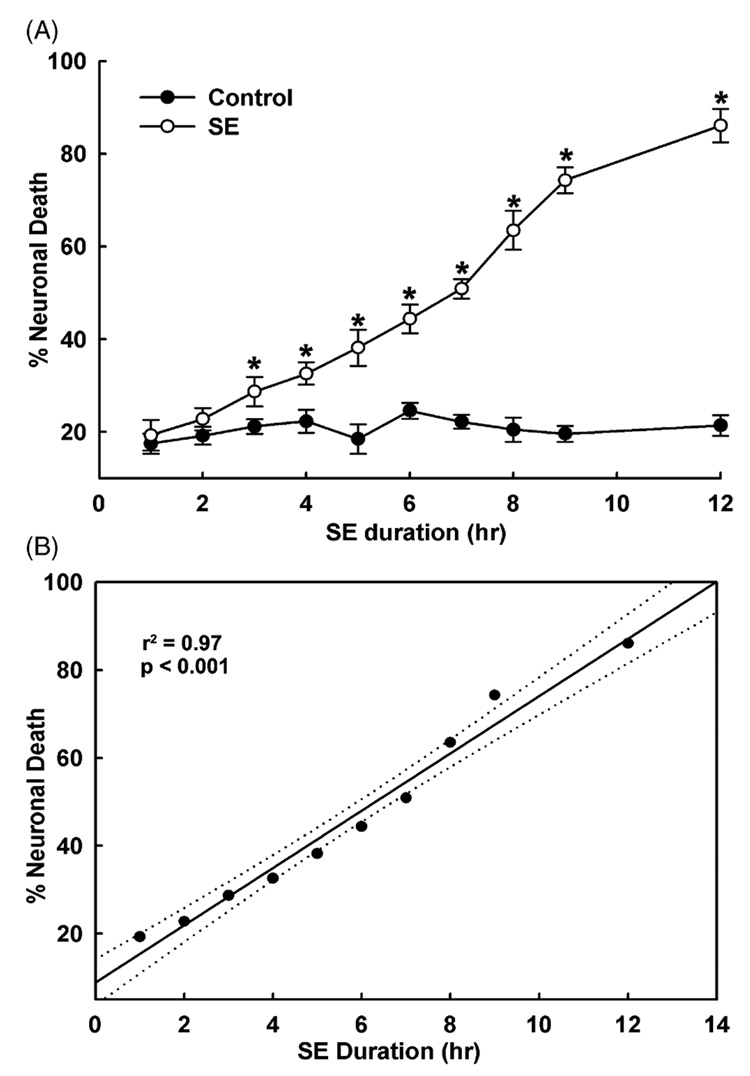
As shown in Fig. 3A, increasing durations of exposure to low Mg2+ caused the percentage of neuronal cell death to increase correspondingly. Cell death increased from approximately 10–15% after 3 h of continuous high-frequency epileptiform discharges to 55–60% after 9 h of exposure to low Mg2+. After 12 h of continuous high-frequency epileptiform discharges, approximately 80% of the neurons manifested cell death. Control cultures represent neuronal cultures subjected to the handling procedures, including removal of media, washing with pBRS and maintenance in pBRS for the same durations that were used for the low Mg2+ time course cultures (section: Hippocampal neuronal culture model of continuous high-frequency epileptiform discharges). Control culture neuronal cell death was assessed at approximately 18%. This amount of cell death represented neuronal injury due to handling effects of the cultures. The cell death values reported in Fig. 3A were adjusted for control cell death in order to reflect net cell death occurring from low Mg2+ treatment alone in the absence of these handling artifacts. Differences in neuronal cell death between control and low Mg2+ conditions were not significant at 0, 1 and 2 h time points, but were significant for low Mg2+ durations of 3–12 h (p < 0.001, Fig. 3A). In order to quantify the correlation of neuronal cell death and duration of continuous high-frequency epileptiform discharges, a linear regression analysis was performed. As shown in Fig. 3B, the percent neuronal cell death correlated significantly with the duration of exposure to low Mg2+ (r2 = 0.972, p < 0.001). These results demonstrate a significant relationship between duration of continuous high-frequency epileptiform discharges and the degree of neuronal cell death.
Figure 3. Correlation between duration of low Mg2+-induced high-frequency spiking and neuronal cell death.
(A) There was a time-dependent increase in neuronal cell death in cultures exposed to increasing durations of low Mg2+-induced high-frequency epileptiform discharges (abbreviated SE in the figure) ranging from 0 to 12 h (n = 6–9 at each time point tested). Black circles (●) indicate sham controls. Open circles (○) represent percent cell death from low Mg2+ exposed cultures. Statistically significant differences were observed for all the time points starting at 3 h following exposure to low Mg2+ solution (*p < 0.001, one-way ANOVA). (B) Linear regression analysis of neuronal cell death and duration of exposure to low Mg2+ solution gave an r2-value of 0.97 (p < 0.001). Dotted lines represent 95% confidence interval limits.
Accumulation of glutamate or other metabolites into the media do not cause neuronal cell death
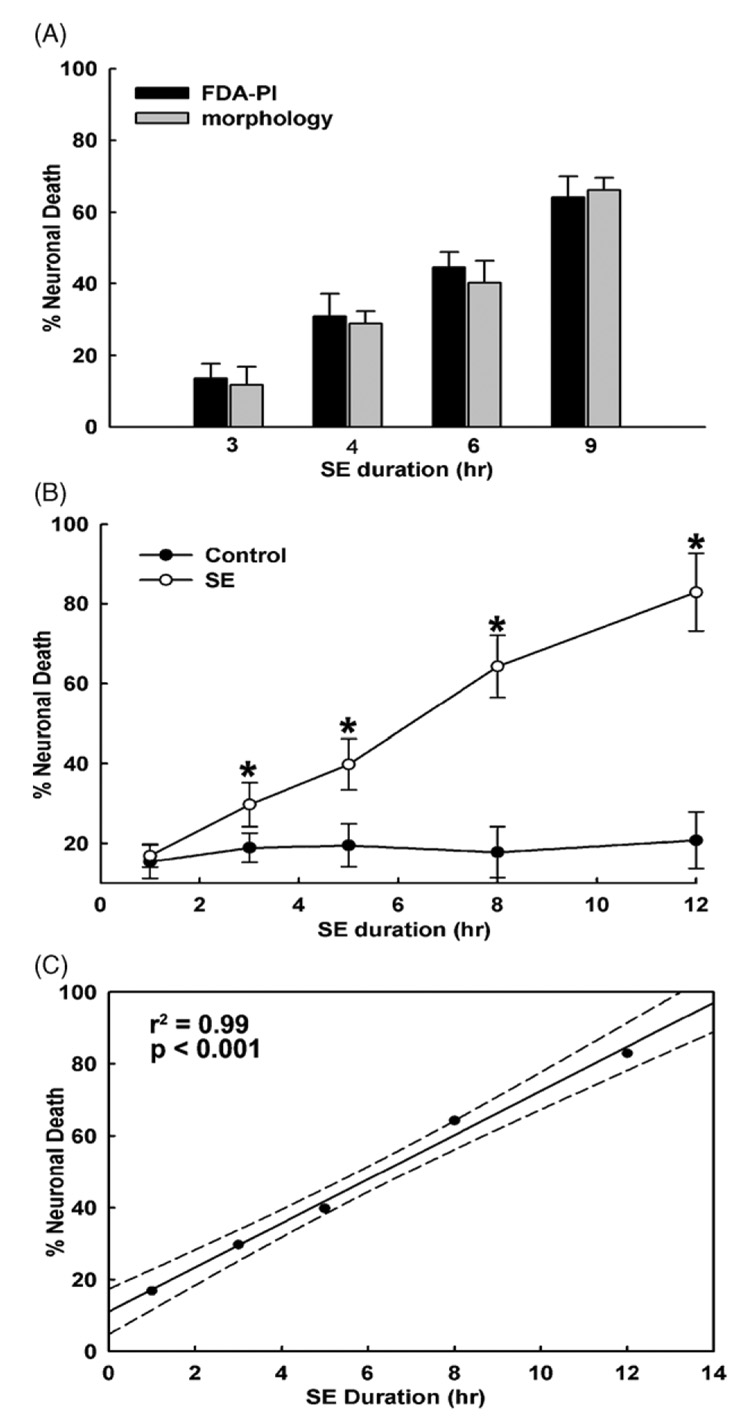
To rule out the possible neurotoxic effects of the accumulation of glutamate or other metabolites in the media on the time-dependent low Mg2+-induced neuronal cell death, we performed experiments wherein fresh low Mg2+ medium was constantly perfused over the culture plate using the gravity feed perfusion apparatus (section: Hippocampal neuronal culture model of continuous high-frequency epileptiform discharges). Glutamate was measured before and during low Mg2+-induced continuous high-frequency spiking (section ‘Materials and methods’). The glutamate concentration in the media alone was not detectable. When replaced with pBRS, the glutamate concentration in neuronal culture was estimated to be less than 1 µmol/l and essentially not detectable. At various time points in the presence of the low Mg2+ solution, the glutamate concentration was measured. The concentration of glutamate steadily rose to a maximal level of 5–6 µmol/l at the longest durations of SE. Upon continuous perfusion with a low Mg2+ solution, the glutamate concentration throughout the duration of experiment was maintained at not detectable levels, less than 1µmol/l. While our perfusion system cannot effectively remove synaptic glutamate, it prevented the build up of glutamate or other materials in the neuronal culture media and thus, maintained a constant environment for the neurons during continuous high-frequency epileptiform discharges. These results demonstrate that we developed a model for observing the effects of continuous epileptiform discharges on neuronal cell death in a carefully controlled neuronal environment. Both control and low Mg2+ treated neuronal cultures were subjected to evaluation using the perfusion technique.
Fig. 4 demonstrates that despite the continuous perfusion of fresh low Mg2+ solution, neuronal cell death increased with increasing durations of continuous high-frequency epileptiform discharges in comparison to control neurons. Similar to the non-perfusion experiments (Fig. 3), both FDA–PI staining and morphological methods demonstrated an increase in neuronal cell death as a function of time (Fig. 4A and B). We did observe a small decrease in neuronal cell death with increasing durations of status epilepticus when fresh low Mg2+ solution was continuously perfused throughout the duration of experiment compared to the non-perfusion conditions. The decrease in neuronal cell death was highest at the longer time points (<3–5%); however, this reduction in cell death did not reach statistical significance. In addition, a significant correlation was also observed between percent neuronal death and duration of SE in the continuous perfusion experiments (r2 = 0.99, p < 0.001, Fig. 4C). These experiments indicate that the continuous electrographic activity alone and not the build up of glutamate or other seizure-released metabolites were responsible for neuronal cell death during low Mg2+-induced continuous epileptiform activity.
Figure 4. Accumulation of glutamate or other metabolites into the media do not cause cell death.
Low Mg2+-induced possible accumulation of glutamate or other metabolite in the culture media was prevented by continuously perfusing culture plate with fresh low Mg2+ solution throughout the duration of experiment using gravity feed perfusion system. (A) Both FDA–PI (black bars) and morphological assessment (gray bars) procedures still revealed a time-dependent increase in neuronal cell death as the result of continuous high-frequency epileptiform discharges (abbreviated SE in the figure), despite continuous perfusion of low Mg2+ perfusion media. (B) Time-dependent increase in neuronal cell death with increasing durations of exposure to low Mg2+ solution despite continuous perfusion of fresh low Mg2+ perfusion media. Low Mg2+ solution was perfused throughout the duration of experiment ranging from 0 to 12h (n = 6–10). Black circles (●) indicate sham controls. Open circles (○) represent percent cell death from low Mg2+ exposed cultures (abbreviated SE in the figure). Both control and low Mg2+ exposed neurons were subjected to similar perfusion protocol. Significant differences were observed for all the time points starting at 3 h following exposure to low Mg2+ solution (*p < 0.001, one-way ANOVA). (C) Linear regression analysis of neuronal cell death and duration of high-frequency epileptiform discharges under constant low Mg2+ perfusion conditions (abbreviated SE in the figure) gave an r2-value of 0.99 (p < 0.001). Dotted lines represent 95% confidence interval limits.
SREDs do not cause cell death
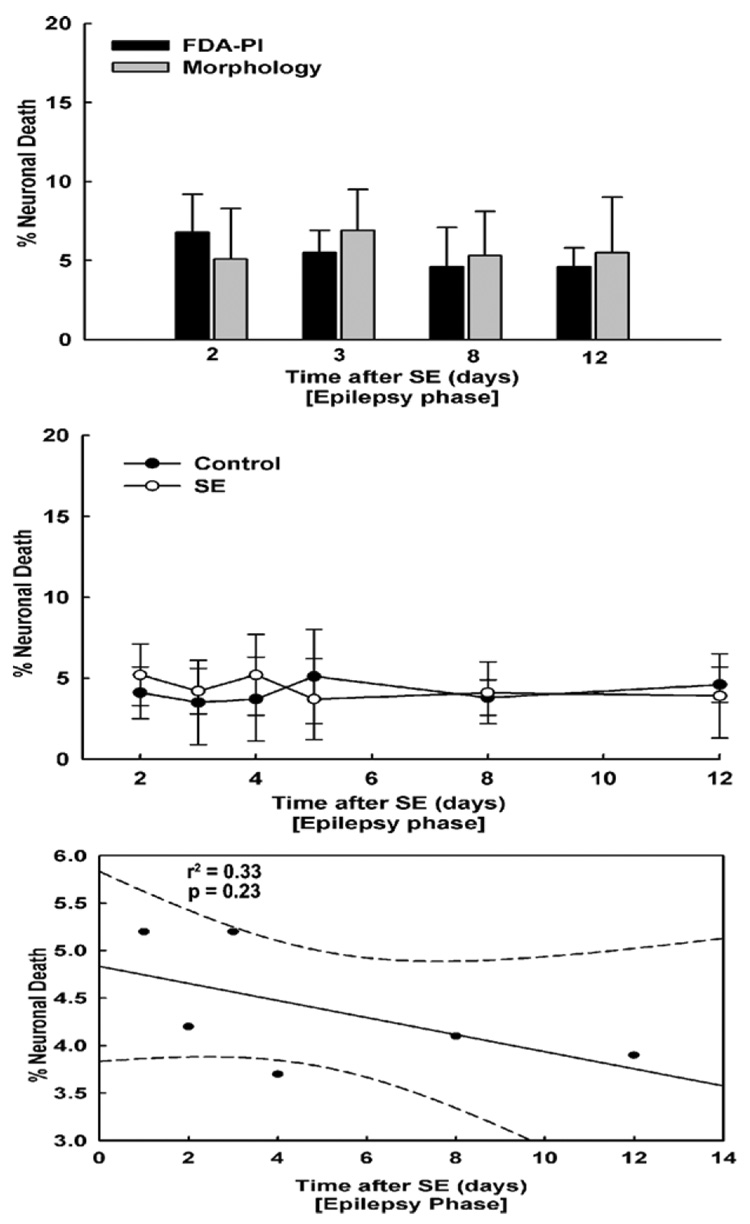
SREDs were induced in neuronal cultures as outlined in section ‘SREDs in hippocampal neuronal cultures’. Before carrying out cell death measurements, neuronal cultures were first confirmed to be manifesting SREDs using patch clamp electrophysiology. SREDs were observed in the cultures at all the time points tested (Fig. 1E and F). To allow the cells to recover from the initial 3 h low Mg2+-induced injury (section ‘Hippocampal neuronal culture model of SREDs’) we conducted experiments starting 2 days after the initial insult from the 3 h of continuous epileptiform discharges. Fig. 5 presents the effects of SREDs in comparison to control cultures on neuronal cell death. Using both the FDA–PI staining and morphological assessment methods, we observed that SREDs did not cause a significant increase in neuronal cell death in comparison to age-matched control cultures (Fig. 5B). Moreover there was no significant correlation between percent neuronal death and time of exposure to SREDs (r2 = 0.26, p = 0.3, Fig. 5C). These experiments demonstrate that spontaneous recurrent epileptiform activity in cell cultures did not cause neuronal cell death. The cell death in age-matched control cultures was 4–6% and this percent cell death was lower than that observed for the control conditions in the pBRS washed cultures due to the lack of washing and handling of the neurons and the fact that we measured cell death in the control culture maintained neurons without manipulation.
Figure 5. Spontaneous recurrent seizures do not cause neuronal cell death.
(A) FDA–PI staining (black bars) and morphological assessment (gray bars) procedures revealed no increase in percent cell death with longer exposure to SREDs. (B) The amount of cell death in SREDs cultures was similar to age-matched control cultures and did not increase with time. Thus there was no increase in neuronal cell death in SREDs phase with time. The cell death in control cultures was around 4–6% and was lower than the control conditions for the pBRS washed cultures due to the lack of washing and handling of the neurons and the direct assessment of the cell death in culture maintained in media without manipulation. (C) Linear regression analysis revealed no significant correlation (r2 = 0.26, p = 0.3) between neuronal cell death and time of exposure to SREDs in cultures.
Discussion
This study provides a direct demonstration in a controlled environment that continuous electrographic epileptiform activity, but not subsequent spontaneous recurrent epileptiform discharges caused cell death in hippocampal neurons in culture. We observed a strong correlation between duration of continuous high-frequency epileptiform activity and subsequent neuronal death. However, there was no significant correlation between the time of exposure to SREDs and neuronal death. In fact, aside from the cell death associated with the initial exposure to low Mg2+, there was no increase in neuronal cell death in cultures manifesting SREDs compared to age-matched control cultures for the life of the neurons in culture. The results are consistent with animal and human data that demonstrate that the majority of neuronal cell death is associated with SE and not with recurrent seizures (Duncan, 2002; Fujikawa, 2005; Fujikawa et al., 2000; Gorter et al., 2003; Holmes, 2002; Pitkanen et al., 2002). To our knowledge this study provides the first direct evidence in an in vitro model that continuous electrographic epileptiform activity is directly correlated with neuronal cell death in the absence of any other extracellular environmental effects on neuronal viability.
SE is a major neurological emergency associated with considerable morbidity and mortality (Delorenzo, 2006). SE is known to cause extensive neuronal damage resulting in further brain abnormalities such as memory deficits and the development of acquired epilepsy (Drislane, 2000). Neuronal injury secondary to SE is a consequence of excessive neuronal excitability and the underlying etiology of SE (Lowenstein and Alldredge, 1998). Thus, it is important to stop ongoing SE and limit neuronal death. Microdialysis studies in humans (During and Spencer, 1993; Wilson et al., 1996) and animals (Smolders et al., 1997; Ueda et al., 2002) have shown significant elevations in extracellular glutamate during SE. On the other hand, there are also many microdialysis studies that failed to show increases in extracellular glutamate during SE (Bruhn et al., 1992; Millan et al., 1993). However it is well established that prolonged exposure to glutamate can kill neurons (Choi et al., 1987; Limbrick et al., 2003) and it has been suggested that increased glutamate in the extracellular space may account for neuronal death associated with SE (Delorenzo et al., 2005; Fountain and Lothman, 1995; Fujikawa, 2005; Wasterlain et al., 1993). While our experiments with continuous perfusion of the extracellular media throughout the duration of high-frequency spiking cannot lower the synaptically trapped glutamate, our measurements show that this system can lower and prevent build up of glutamate in the extracellular media. Under these conditions the possible neurotoxic effects of extracellular accumulation of glutamate and other substances are eliminated, providing a well-controlled environment to conduct these studies. The cell death that occurs under these perfusion conditions is therefore a result of excessive synaptic activity. These findings are further supported by the observation that prolonged status epilepticus such as caused by the electrical stimulation of the perforant pathway and excessive synaptic activity can damage postsynaptic neurons (Olney et al., 1983; Sloviter, 1983). Taken together, these results further stress the importance of limiting seizure duration during SE to prevent brain injury.
Data from animal studies on the effects of SE and seizures on neuronal cell death demonstrate results similar to the human studies (Fujikawa, 2005; Holmes, 2002). In vivo studies utilizing rat models of SE-induced temporal lobe epilepsy suggest that neuronal cell death is only associated with the initial injury from SE, but not with the subsequent spontaneous recurrent seizures (Gorter et al., 2003; Pitkanen et al., 2002); however, there is data showing that even a few evoked seizures in kindled rats produce TUNEL-positive hippocampal in hilar neurons (Bengzon et al., 1997) and hippocampal neuronal loss that increases with increasing numbers of kindled seizures (Cavazos et al., 1994). Clinical studies have demonstrated conflicting results with reports of increased serum and CSF levels of neuron-specific enolase, a marker of neuronal injury, after spontaneous seizures (Buttner et al., 1999). Recently, it was shown in a population-based, longitudinal quantitative MRI study of 179 patients with epilepsy that progressive regional or global cerebral damage was not a consequence of epileptic seizures (Liu et al., 2005). Furthermore, a separate quantitative post-mortem study of the human hippocampus in chronic epilepsy showed that seizures do not inevitably cause neuronal loss (Thom et al., 2005). Our study supports these findings and demonstrates that spontaneous recurrent seizures occurring several times an hour for durations of up to 10 days did not produce neuronal death in comparison to matched control cultures. These in vitro studies support the in vivo animal and human studies indicating that epileptic seizures do not cause neuronal damage by themselves.
Determining the effects of recurrent seizures in humans or animals is complicated by several factors such as age, type of convulsant stimuli or seizure type, treatment with antiepileptic drugs and numerous other clinical variables. The results from this study demonstrate that the hippocampal neuronal culture model provides a powerful in vitro model that allows for carefully evaluating the effects of electrographic epileptiform activity similar to SE and epilepsy on neuronal viability in the absence of various confounding factors. It will be interesting to further investigate mechanisms of neuronal cell death during SE in this controlled environment in future studies. Understanding the molecular mechanisms mediating neuronal death due to continuous epileptiform may provide new insights into the development of novel therapeutic agents to prevent neuronal injury during SE.
Acknowledgements
National Institute of Neurological Disorders and Stroke grants RO1NS051505, RO1NS052529 and UO1NS058213; the Milton L. Markel Alzheimer’s Disease Research Fund; and the Sophie and Nathan Gumenick Neuroscience Research Fund to RJD supported this work. We thank Drs. M. Ross Bullock and Oscar L. Alves for their assistance with glutamate concentration measurements and Elisa Attkisson for her help with preparation and maintenance of neuronal cultures.
References
- Bengzon J, Kokaia Z, Elmer E, Nanobashvili A, Kokaia M, Lindvall O. Apoptosis and proliferation of dentate gyrus neurons after single and intermittent limbic seizures. Proc. Natl. Acad. Sci. U.S.A. 1997;94:10432–10437. doi: 10.1073/pnas.94.19.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RE, Deshpande LS, Sombati S, Falenski KW, Martin BR, DeLorenzo RJ. Activation of the cannabinoid type-1 receptor mediates the anticonvulsant properties of cannabinoids in the hippocampal neuronal culture models of acquired epilepsy and status epilepticus. J. Pharmacol. Exp. Ther. 2006;317:1072–1078. doi: 10.1124/jpet.105.100354. [DOI] [PubMed] [Google Scholar]
- Bruhn T, Cobo M, Berg M, Diemer NH. Limbic seizure-induced changes in extracellular amino acid levels in the hippocampal formation: a microdialysis study of freely moving rats. Acta Neurol. Scand. 1992;86:455–461. doi: 10.1111/j.1600-0404.1992.tb05123.x. [DOI] [PubMed] [Google Scholar]
- Buttner T, Lack B, Jager M, Wunsche W, Kuhn W, Muller T, Przuntek H, Postert T. Serum levels of neuron-specific enolase and s-100 protein after single tonic-clonic seizures. J. Neurol. 1999;246:459–461. doi: 10.1007/s004150050383. [DOI] [PubMed] [Google Scholar]
- Cavazos JE, Das I, Sutula TP. Neuronal loss induced in limbic pathways by kindling: evidence for induction of hippocampal sclerosis by repeated brief seizures. J. Neurosci. 1994;14:3106–3121. doi: 10.1523/JNEUROSCI.14-05-03106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW, Maulucci-Gedde M, Kriegstein AR. Glutamate neurotoxicity in cortical cell culture. J. Neurosci. 1987;7:357–368. doi: 10.1523/JNEUROSCI.07-02-00357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churn S, Limbrick D, Sombati S, DeLorenzo R. Excitotoxic activation of the NMDA receptor results in inhibition of calcium/calmodulin kinase II activity in cultured hippocampal neurons. J. Neurosci. 1995;15:3200–3214. doi: 10.1523/JNEUROSCI.15-04-03200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churn SB, Sombati S, Jakoi ER, Sievert L, DeLorenzo RJ. Inhibition of calcium/calmodulin kinase II alpha subunit expression results in epileptiform activity in cultured hippocampal neurons. Proc. Natl. Acad. Sci. U.S.A. 2000;97:5604–5609. doi: 10.1073/pnas.080071697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorenzo RJ. Epidemiology and clinical presentation of status epilepticus. Adv. Neurol. 2006;97:199–215. [PubMed] [Google Scholar]
- Delorenzo RJ, Sun DA, Deshpande LS. Cellular mechanisms underlying acquired epilepsy: the calcium hypothesis of the induction and maintainance of epilepsy. Pharmacol. Ther. 2005;105:229–266. doi: 10.1016/j.pharmthera.2004.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande LS, Blair RE, Nagarkatti N, Sombati S, Martin BR, Delorenzo RJ. Development of pharmacoresistance to benzodiazepines but not cannabinoids in the hippocampal neuronal culture model of status epilepticus. Exp. Neurol. 2007a doi: 10.1016/j.expneurol.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande LS, Blair RE, Ziobro JM, Sombati S, Martin BR, DeLorenzo RJ. Endocannabinoids block status epilepticus in cultured hippocampal neurons. Eur. J. Pharmacol. 2007b;558:52–59. doi: 10.1016/j.ejphar.2006.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande LS, Sombati S, Blair RE, Carter DS, Martin BR, DeLorenzo RJ. Cannabinoid CB1 receptor antagonists cause status epilepticus-like activity in the hippocampal neuronal culture model of acquired epilepsy. Neurosci. Lett. 2007c;411:11–16. doi: 10.1016/j.neulet.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didier M, Heaulme M, Soubrie P, Bockaert J, Pin JP. Rapid, sensitive, and simple method for quantification of both neurotoxic and neurotrophic effects of NMDA on cultured cerebellar granule cells. J. Neurosci. Res. 1990;27:25–35. doi: 10.1002/jnr.490270105. [DOI] [PubMed] [Google Scholar]
- Drislane FW. Presentation, evaluation, and treatment of nonconvulsive status epilepticus. Epilepsy Behav. 2000;1:301–314. doi: 10.1006/ebeh.2000.0100. [DOI] [PubMed] [Google Scholar]
- Duncan JS. Seizure-induced neuronal injury: human data. Neurology. 2002;59:15S–20S. doi: 10.1212/wnl.59.9_suppl_5.s15. [DOI] [PubMed] [Google Scholar]
- During MJ, Spencer DD. Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet. 1993;341:1607–1610. doi: 10.1016/0140-6736(93)90754-5. [DOI] [PubMed] [Google Scholar]
- Fountain NB. Status epilepticus: risk factors and complications. Epilepsia. 2000;41(Suppl 2):S23–S30. doi: 10.1111/j.1528-1157.2000.tb01521.x. [DOI] [PubMed] [Google Scholar]
- Fountain NB, Lothman EW. Pathophysiology of status epilepticus. J. Clin. Neurophysiol. 1995;12:326–342. [PubMed] [Google Scholar]
- Fujikawa DG. The temporal evolution of neuronal damage from pilocarpine-induced status epilepticus. Brain Res. 1996;725:11–22. doi: 10.1016/0006-8993(96)00203-x. [DOI] [PubMed] [Google Scholar]
- Fujikawa DG. Prolonged seizures and cellular injury: understanding the connection. Epilepsy Behav. 2005;7:3–11. doi: 10.1016/j.yebeh.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Fujikawa DG, Itabashi HH, Wu A, Shinmei SS. Status epilepticus-induced neuronal loss in humans without systemic complications or epilepsy. Epilepsia. 2000;41:981–991. doi: 10.1111/j.1528-1157.2000.tb00283.x. [DOI] [PubMed] [Google Scholar]
- Goodkin HP, Yeh J-L, Kapur J. Status epilepticus increases the intracellular accumulation of GABAA receptors. J. Neurosci. 2005;25:5511–5520. doi: 10.1523/JNEUROSCI.0900-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorter JA, Pereira PMG, van Vliet EA, Aronica E, da Silva FHL, Lucassen PJ. Neuronal cell death in a rat model for mesial temporal lobe epilepsy is induced by the initial status epilepticus and not by later repeated spontaneous seizures. Epilepsia. 2003;44:647–658. doi: 10.1046/j.1528-1157.2003.53902.x. [DOI] [PubMed] [Google Scholar]
- Holmes GL. Seizure-induced neuronal injury: animal data. Neurology. 2002;59:3S–6S. doi: 10.1212/wnl.59.9_suppl_5.s3. [DOI] [PubMed] [Google Scholar]
- Limbrick DD, Churn SB, Sombati S, DeLorenzo RJ. Inability to restore resting intracellular calcium levels as an early indicator of delayed neuronal cell death. Brain Res. 1995;690:145–156. doi: 10.1016/0006-8993(95)00552-2. [DOI] [PubMed] [Google Scholar]
- Limbrick DD, Jr, Sombati S, DeLorenzo RJ. Calcium influx constitutes the ionic basis for the maintenance of glutamate-induced extended neuronal depolarization associated with hippocampal neuronal death. Cell Calcium. 2003;33:69–81. doi: 10.1016/s0143-4160(02)00054-4. [DOI] [PubMed] [Google Scholar]
- Liu RSN, Lemieux L, Bell GS, Sisodiya SM, Bartlett PA, Shorvon SD, Sander JWAS, Duncan JS. Cerebral damage in epilepsy: a population-based longitudinal quantitative MRI study. Epilepsia. 2005;46:1482–1494. doi: 10.1111/j.1528-1167.2005.51603.x. [DOI] [PubMed] [Google Scholar]
- Lowenstein DH, Alldredge BK. Status epilepticus. N. Engl. J. Med. 1998;338:970–976. doi: 10.1056/NEJM199804023381407. [DOI] [PubMed] [Google Scholar]
- Mangan PS, Kapur J. Factors underlying bursting behavior in a network of cultured hippocampal neurons exposed to zero magnesium. J. Neurophysiol. 2004;91:946–957. doi: 10.1152/jn.00547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum BS, Vigouroux RA, Brierley JB. Systemic factors and epileptic brain damage. Prolonged seizures in paralyzed, artificially ventilated baboons. Arch. Neurol. 1973;29:82–87. doi: 10.1001/archneur.1973.00490260026003. [DOI] [PubMed] [Google Scholar]
- Men SH, Lee DR, Barron FJG, Munoz D. Selective neuronal necrosis associated with status epilepticus: MR findings. Am. J. Neuroradiol. 2000;21:1837–1840. [PMC free article] [PubMed] [Google Scholar]
- Millan MH, Chapman AG, Meldrum BS. Extracellular amino acid levels in hippocampus during pilocarpine-induced seizures. Epilepsy Res. 1993;14:139–148. doi: 10.1016/0920-1211(93)90018-3. [DOI] [PubMed] [Google Scholar]
- Olney JW, deGubareff T, Sloviter RS. ‘‘Epileptic’’ brain damage in rats induced by sustained electrical stimulation of the perforant path. II. Ultrastructural analysis of acute hippocampal pathology. Brain Res. Bull. 1983;10:699–712. doi: 10.1016/0361-9230(83)90038-2. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Nissinen J, Nairismagi J, Lukasiuk K, Grohn OH, Miettinen R, Kauppinen R. Progression of neuronal damage after status epilepticus and during spontaneous seizures in a rat model of temporal lobe epilepsy. Prog. Brain Res. 2002;135:67–83. doi: 10.1016/S0079-6123(02)35008-8. [DOI] [PubMed] [Google Scholar]
- Schreiber SS, Sun N, Tocco G, Baudry M, DeGiorgio CM. Expression of neuron-specific enolase in adult rat brain following status epilepticus. Exp. Neurol. 1999;159:329–331. doi: 10.1006/exnr.1999.7147. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. ‘‘Epileptic’’ brain damage in rats induced by sustained electrical stimulation of the perforant path. I. Acute electrophysiological and light microscopic studies. Brain Res. Bull. 1983;10:675–697. doi: 10.1016/0361-9230(83)90037-0. [DOI] [PubMed] [Google Scholar]
- Smolders I, Khan GM, Manil J, Ebinger G, Michotte Y. NMDA receptor-mediated pilocarpine-induced seizures: characterization in freely moving rats by microdialysis. Br. J. Pharmacol. 1997;121:1171–1179. doi: 10.1038/sj.bjp.0701231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sombati S, DeLorenzo RJ. Recurrent spontaneous seizure activity in hippocampal neuronal networks in culture. J. Neurophysiol. 1995;73:1706–1711. doi: 10.1152/jn.1995.73.4.1706. [DOI] [PubMed] [Google Scholar]
- Sun DA, Sombati S, DeLorenzo RJ. glutamate injury-induced epileptogenesis in hippocampal neurons: an in vitro model of stroke-induced ‘‘Epilepsy’’. Stroke. 2001;32:2344–2350. doi: 10.1161/hs1001.097242. [DOI] [PubMed] [Google Scholar]
- Thom M, Zhou J, Martinian L, Sisodiya S. Quantitative post-mortem study of the hippocampus in chronic epilepsy: seizures do not inevitably cause neuronal loss. Brain. 2005;128:1344–1357. doi: 10.1093/brain/awh475. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Yokoyama H, Nakajima A, Tokumaru J, Doi T, Mitsuyama Y. Glutamate excess and free radical formation during and following kainic acid-induced status epilepticus. Exp. Brain Res. 2002;147:219–226. doi: 10.1007/s00221-002-1224-4. [DOI] [PubMed] [Google Scholar]
- Wasterlain CG, Fujikawa DG, Penix L, Sankar R. Pathophysiological mechanisms of brain damage from status epilepticus. Epilepsia. 1993;34(Suppl 1):S37–S53. doi: 10.1111/j.1528-1157.1993.tb05905.x. [DOI] [PubMed] [Google Scholar]
- Wilson CL, Maidment NT, Shomer MH, Behnke EJ, Ackerson L, Fried I, Engel J. Comparison of seizure related amino acid release in human epileptic hippocampus versus a chronic, kainate rat model of hippocampal epilepsy. Epilepsy Res. 1996;26:245–254. doi: 10.1016/s0920-1211(96)00057-5. [DOI] [PubMed] [Google Scholar]