Abstract
Alanine aminotransferase (ALT) is a key enzyme for gluconeogenesis as well as a widely-used serum marker for liver injury. We have identified two ALT isoenzymes, ALT1 and ALT2, which are encoded by separate genes. In this study, we described the expression, purification and initial characterization of human ALT1 and ALT2 proteins in High-five insect cells. Human ALT1 and ALT2 were expressed as His-tagged fusion proteins by recombinant baculovirus in insect cells and purified into homogeneity in one step by using immobilized Ni2+-affinity chromatography. Tag-free ALT1 and ALT2 were obtained by cleavage of enterokinase digestion and used for initial characterization of the enzymes. The specific ALT activity of purified fusion or His-tag-removed ALT1 is about 15-fold higher than that of ALT2 and their enzymatic activities decrease quickly at 37°C and −20°C, but are well preserved at −80°C. Nevertheless, the ALT1 and ALT2 activities remained stable in a buffer containing 25% glycerol. The pH profile was similar between hALT1 and hALT2 in that both enzymes remained fully active between pH 6.5 and 8.0. The purified ALT recombinant proteins can not only be used as a reference protein standard for the ALT assay in clinical chemistry, but also will be useful for understanding biochemical and biological significance of the isoenzymes and for developing the ALT isoform-specific assays for clinical or preclinical diagnostic use.
Alanine aminotransferase (ALT) [EC 2.6.1.2., formerly glutamate-pyruvate transaminase], is a pyridoxal enzyme catalyzing reversible transamination between alanine and 2-oxoglutarate to form pyruvate and glutamate. Through producing those four important intermediary metabolites, ALT plays an important role in gluconeogenesis and amino acid metabolism, particularly during fasting and exercise where glucose is made from pyruvate converted from alanine through de-transamination by ALT [1].
Recently, two isoforms of ALT, ALT1 and ALT2, each encoded by a different gene, have been identified in human and mouse with distinct tissue expression at the mRNA level; ALT1 is mainly expressed in intestine, liver, kidney, and heart, whereas ALT2 is mainly expressed in muscle, brain, fat, kidney and liver [2, 3], suggesting a functional difference in biology between the isoenzymes. On the other hand, the measurement of serum ALT activity is one of the most widely-used assays in clinical chemistry. An elevated serum ALT activity is regarded as evidence of liver damage, including hepatitis, nonalcoholic steatohepatosis, fatty liver, cirrhosis, and drug hepatoxicity [4–6]. But serum ALT elevation is also observed in extra-hepatic diseases, such as muscle disease and obesity [7, 8], and in apparently healthy people [9, 10]. The difference of ALT isoenzymes in structure, tissue and subcellular distributions, and gene regulation suggests that the two isoforms may have different biological functions and may behave discordantly under various clinical situations. Thus, measurement of ALT isoforms may have more diagnostic value than the currently-being-used ALT activity assay, which presumably measure total ALT1 and ALT2 activity. Currently, ALT catalytic activity is measured in clinical chemistry by a spectrophotometric assay, whose accuracy is vulnerable to the influence of a numbers of factors including temperature, substrate concentration and sample handling and storage conditions [11]. The variations of ALT activity assays among and within the laboratories and the lack of a protein-based reference have made it difficult to define a “normal” range of serum ALT levels in healthy people and hence, a standardization of ALT assays is recommended [11, 12]. We had previously expressed human ALT isoforms in bacteria [2]. Unfortunately, most of the recombinant proteins were insoluble, preventing us from obtaining sufficient amounts of the proteins in native form for functional characterization and high-affinity antibody production. Canine ALT1 has been purified in soluble form in E. Coli cells grown at a reduced (20 °C) temperature [13], but it is not ideal for clinical chemistry studies in humans because of less conservation of the protein between the two species. Purification of recombinant human ALT1 and ALT2 will not only facilitate the understanding of their biological significance, but also provide a valuable ALT reference for clinical chemistry. The baculovirus-insect cell system has been used successfully for the expression of thousands of diverse types of proteins in soluble and functional form [14]. Thus, we expressed, purified the recombinant hALT1 and hALT2 in insect cells, and did preliminarily characterization of the two isoenzymes.
Materials and methods
Vector construction
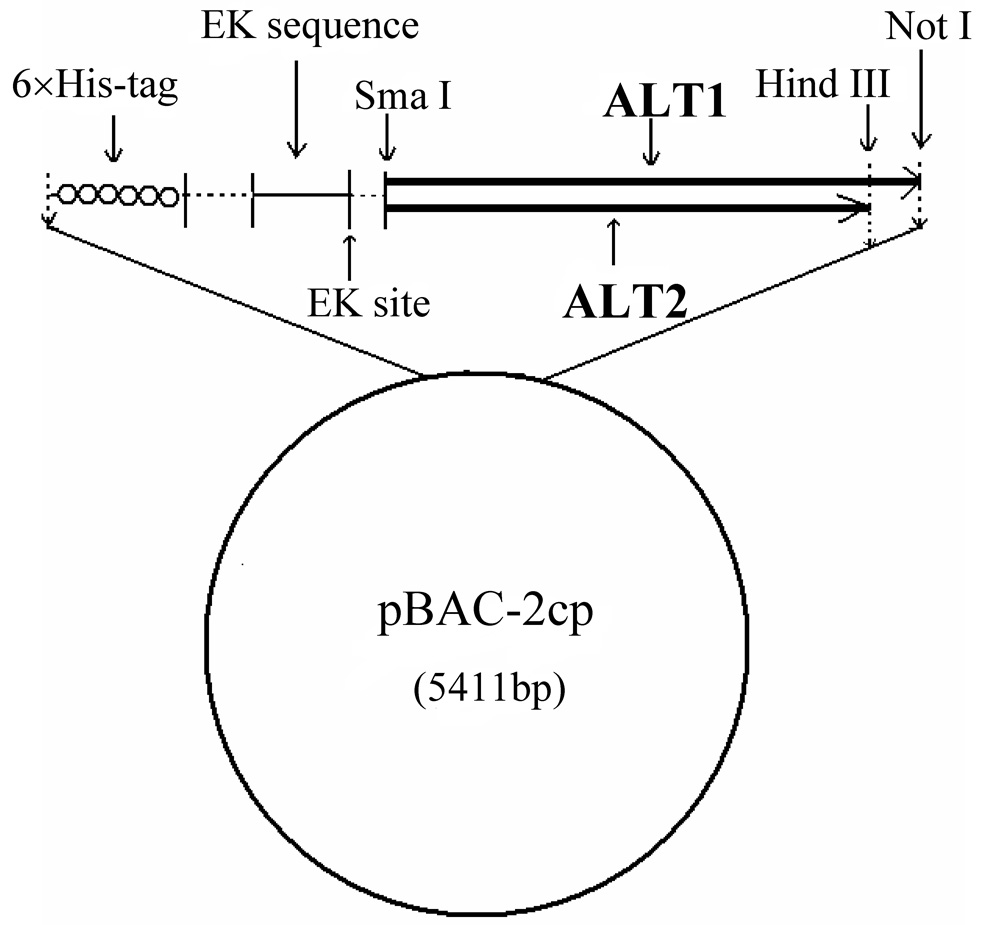
The full-length cDNA of human ALT1 was excised from ALT1-pET28a [2] by Nde I (blunted) and Not I and ligated into Sma I/Not I-cut pBAC-2cp vector (Novagen, San Diego, CA). The resulting plasmid was sequenced to confirm no cloning-introduced mutations. The final construct that encodes 6 x histidine tag (His-tag), enterokinase cleavage site and human ALT1 (from the N- to C-terminus) is referred to as pBAC-2cp-hALT1 (Fig. 1).
Fig. 1.
Schematic diagram of vector construction for human ALT1 and ALT2 recombinant baculovirus generation. Human ALT1 or ALT2 cDNA is subcloned in frame with 6 x His-tag and enterokinase sequences of the baculovirus transfer vector pBAC-2cp
For human ALT2 cloning, human liver total RNA (Clontech, Mountain View, CA ) was converted into first-strand cDNA by using a reverse transcription system (Promega, Madison, WI). The coding sequence of human ALT2 without the mitochondrial signal sequence, was amplified from the first-strand cDNA by the polymerase chain reaction (35 cycles of 98°C for 10 sec, 55°C for 30 sec, and 72°C for 1 min 30 sec with final extension of 7 min at 72°C) using Phusion High-Fidelity PCR kit (New England Biolabs, Ipswich, MA) with a forward Sma I linker primer (5’-ctctcccgggaGCCGAGGCCTCGGCGGTGC-3’; lower case, linker sequence; upper case, gene-specific sequence), and a reverse Hind III linker primer (5’-ggctcagaagcttTCACGCGTACTTCTCCAGGAAGTTGATGTGG-3’). The resulting PCR product was sub-cloned into pCR-BluntII-TOPO (Invitrogen) vector, referred as hALT2-TOPO. The absence of PCR-derived mutations in the inserted human ALT2 cDNA was verified by DNA sequence analysis. Then human ALT2 cDNA was excised from hALT2-TOPO with Sma I/Hind III restriction enzymes, sub-cloned into pBAC-2cp vector and referred to as pBAC-2cp-hALT2 (Fig.1).
Generation of human ALT1 and ALT2 recombinant baculoviruses in Sf9 insect cells
A similar protocol was used to express and purify recombinant hALT1 and hALT2 proteins. Spodoptera frugiperda Sf9 insect cells were grown in TNM-FH medium (SAFC Biosciences, Kansas, USA) supplemented with 10% FBS and 50 ug/ml gentamicin at 27°C. To generate ALT baculovirus, 1.5 µg of pBAC-2cp-hALT1 or pBAC-2cp-hALT2 was transfected into Sf9 insect cells with 200 ng of linearized BaculoGold Bright DNA (BD Biosciences, San Diego, CA) using Cellfectin (Invitrogen) transfection reagent according to the manufacturer’s protocol. 4–5 days after transfection, recombinant baculoviruses were harvested from the cell culture medium and stored at 4°C and named generation 1 virus, which was sequentially amplified by infecting more Sf9 cells until generation 4. As the recombinant virus carries green fluorescence protein (GFP), the virus titer was determined by the method of end point dilution through counting the numbers of infected green cells under fluorescence microscopy 48 hours after infection [15].
Expression and purification of recombinant ALT proteins in High-five insect cells
150 ml of High-five insect cells (1~2×106 cells/ml, Invitrogen) were grown in Ex-cell 405 medium (SAFC Biosciences) and infected by the generation 4 virus at a multiplicity of infection (MOI) of 5 to express human ALT1 and ALT2 recombinant proteins by shaking (150 rpm/min) at 27°C. 48 hours after the infection, cells were harvested by centrifugation at 1000 rpm for 10 min at 4°C and the pellet was resuspended in 30 ml of buffer A (20 mM sodium phosphate, pH 7.4 and 0.5 M sodium chloride), and disrupted with a Fisher sonicator on ice (setting: strength 4, 20 seconds each for 12 times). Cell lysates were centrifuged at 50,000 rpm at 4°C in a Beckman Ti 90 rotor for 30 min, and the supernatant was passed through 0.22 µm filter before being applied to a 5 ml HiTrap chelating HP column (Amersham Biosciences, Piscataway, NJ) that had been equilibrated with buffer A at a flow rate of 1 ml/min on AKTA 10 FPLC system (Amersham Bioscience). The column-bound proteins were eluted with a linear gradient to 100% buffer B (buffer A with 500 mM imidazole) at a flow rate of 1 ml/min. Fractions from 0 to 500 mM imidazole were collected and analyzed by SDS-10% polyacrylamide gel electrophoresis (PAGE), ALT activity assay, Coomassie blue staining, or Western blotting.
Removal of His-tag from recombinant fusion protein
According to the result of Coomassie blue staining and Western blotting, the peak fractions were pooled together and dialyzed overnight at 4°C against Tris-HCl buffer (20 mM Tris-HCl, pH7.5) in a dialysis tube with a molecular weight cutoff at 10 kDa. Protein concentrations were quantified by Bradford method (Bio-Rad, Hercules, CA) with bovine serum albumin as standard. The recombinant protein was digested by bovine enterokinase (EK) [250 µg protein digested by 50 ng EK [Yuan, 2002 #775] in a buffer containing 20 mM Tris-HCl, pH 7.5, 50 mM NaCl] for 24 hours at room temperature. The digested protein mixture was subjected Ni-NTA agarose chromatography (Qiagen) and the His-tag free ALT was separated from the His-tag fusion protein by a step-wise elution of imidazole from 0 to 320 mM.
Immunoblot analysis
Protein samples were boiled for 5 min in 1 × SDS sample buffer (50 mM Tris-HCl pH 6.8, 12.5% glycerol, 1% SDS, 0.01% bromophenol blue) containing 5% β-mercaptoethanol. Samples were subjected to SDS-10% PAGE. The resolved proteins were transferred for 3 hours at 4°C onto a polyvinylidene difluoride (PVDF) membrane, which was then incubated with mouse anti-His-tag monoclonal antibody (1:3000 dilution, Amersham Biosciences) at 4°C overnight. Next, the blot was washed and probed with the corresponding horseradish peroxidase-conjugated secondary antibodies (1:5000 dilution, Pierce, Rockford, IL) for 45 min at room temperature. Enhanced chemiluminescence (ECL) was used to obtain the Western blot signals.
Enzyme activity assays
ALT activity was measured at room temperature with a HTS7000 assay system according to the following principle: pyruvate produced from the transamination of alanine is quantitatively determined by the LDH-NADH reaction. NADH is oxidized to NAD with a decrease in the absorbance at 340 nm. Therefore, rate of NADH decrease is directly proportional to that of formation of pyruvate and thus the ALT activity. One unit of ALT activity is defined as the amount of enzyme that catalyzes the formation of one micromole per liter of NAD per minute at 25°C.
Effect of temperature and storage conditions on ALTs
To study the effect of temperature and storage conditions on ALT activity, the purified recombinant hALT1 (final, 1.35 µg/ml) and hALT2 proteins (final, 20 µg/ml) were aliquoted into Tris-HCl buffer (20 mM Tris-HCl, pH 7.5). The aliquots were stored at −80°C, −20°C, 4°C, 25°C and 37°C for the indicated periods of time and assayed for enzymatic activity, respectively. To investigate the glycerol effect, purified recombinant hALT1 and hALT2 proteins were added to various concentrations of glycerol (0–30 %) in 20 mM Tris-HCl buffer, pH7.5 at −20°C for 24 and 48 hours respectively. Assays were conducted in triplicate for each point and an average activity was taken.
Effect of pH on ALT activity
The activities of recombinant hALT1 and hALT2 were determined in the pH range from 5.0 to 9.0 (125 mM Tris-HCl) in the presence of 575 mM alanine and 16.3 mM 2-ketoglutarate and contained recombinant ALT1 (1.35 µg/ml) and ALT2 (20 µg/ml) respectively. The pH values were accurately adjusted by pH meter and no pH change was observed during the assay by pH strip measurement. Assays were conducted in triplicate for each point.
Results
Expression and purification of recombinant human ALT1 and ALT2 in High-five insect cells
Previously, we had expressed recombinant hALT1 and hALT2 in bacteria [2], but the level of soluble proteins were too low to be purified for enzymatic characterization. Thus, we decided to utilize insect cells to express the proteins. As purification strategy was similar for both hALT1 and hALT2, we use ALT1 to exemplify the expression and purification process, as described below.
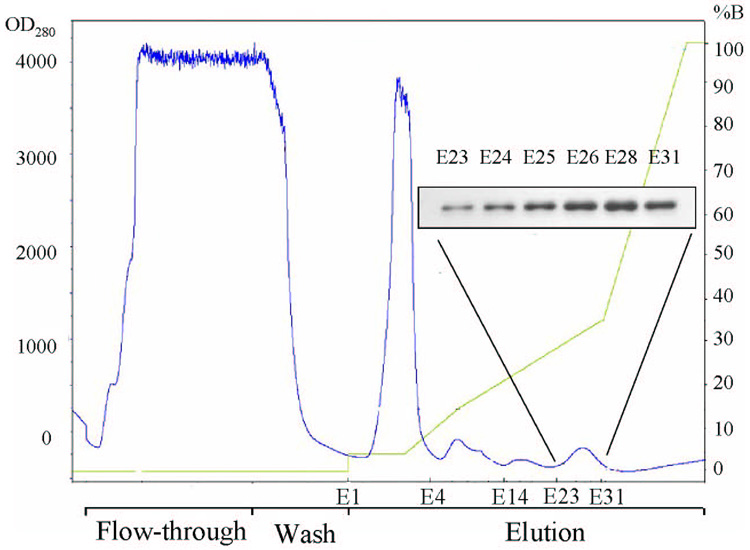
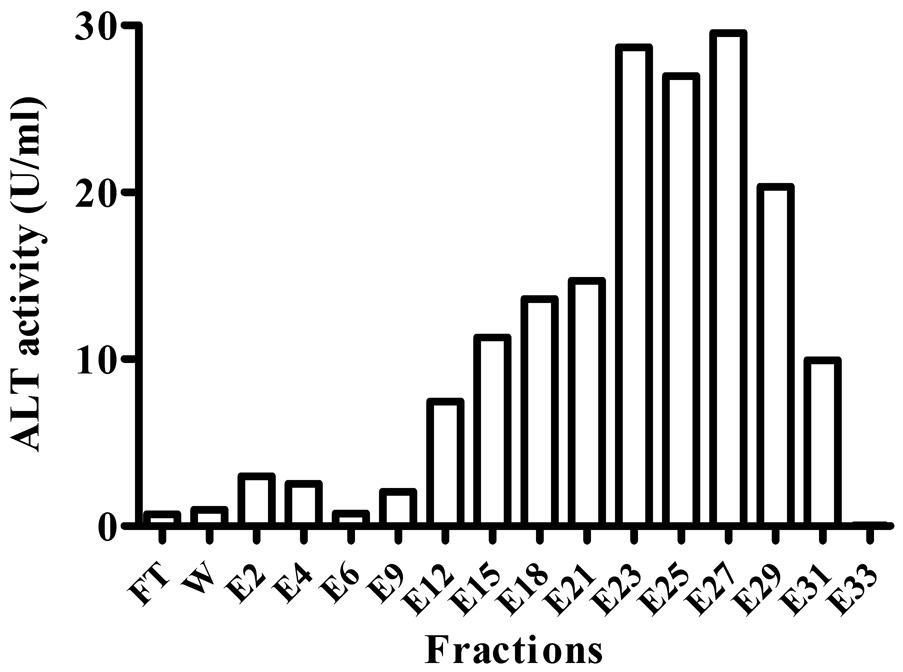
hALT1 over-expressing cell lysates made from High-five insect cells infected with hALT1 baculovirus were subjected to Ni2+-chelating column. Most of protein lysates were not retained on the column, as evidenced by the high absorbance at the OD280 in the flow-through fractions (Fig. 2A). After washing, several small protein peaks were eluted with the increasing gradient of imidazole (0–175 mM) and were assayed for ALT activity. Most of the input ALT activity was recovered in the eluents between 20% and 35 % of buffer B (between 100 mM and 175 mM imidazole, eluents E12–E31, Fig. 2B). These fractions were subsequently analyzed by SDS-PAGE and Western blotting. SDS-PAGE showed that fractions of E23 to E31 were nearly homogenous, with the molecular weight about 60 kDa, very close to the estimated molecular weight of 59 kDa of the fusion protein (Fig. 2C). Positive Western blotting signals in the last peak (Eluents E23–E31) with anti-His-tag antibody confirmed that the recombinant hALT1 is His-tagged (Fig. 2A, Insert).
Fig. 2.
Representative purification of recombinant hALT1. 30 ml of supernatant from hALT1-baculovirus-infected High-five cells was subjected to Ni2+-loaded HiTrap chelating HP column (5 ml), washed with 25 ml of Buffer A and eluted with a gradient of Buffer B. Protein elution was monitored at the absorbance of OD280 nm (Fig. 2A). The fractions of flow-through (FT, pooled), wash (W, pooled) and elution (E) were measured for ALT activity (Fig. 2B). Fractions during the purification were subjected to SDS-10% PAGE and stained with Coomassie blue (Fig 2C). Lane 1, ladder of protein molecular mass marker; 2, uninfected High-five cell supernatant; 3, input; 4, flow-through; 5, wash; Lane 6 to 15, eluents of E4, 6, 9, 14, 16, 18, 21, 23, 26 and 31. Homogenous fractions (E23 to E31) were analyzed by Western blotting with anti-His-tag antibody (Fig 2A, insert).
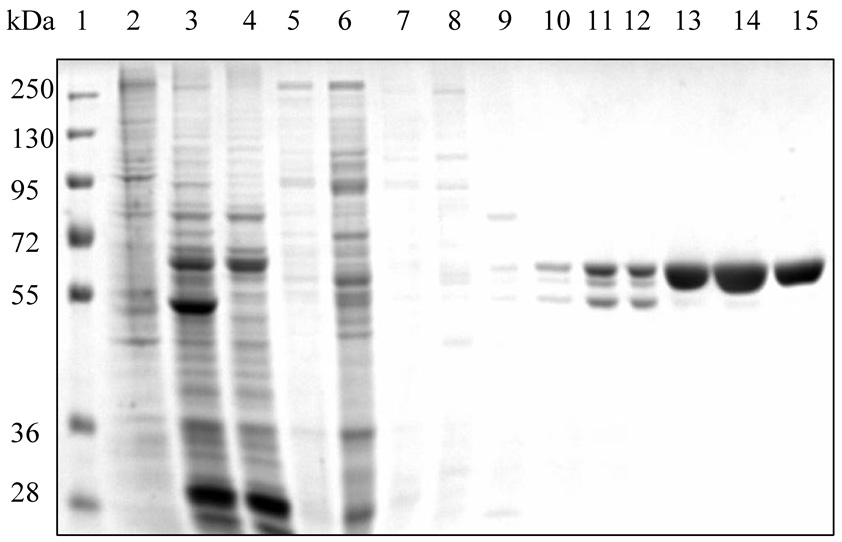
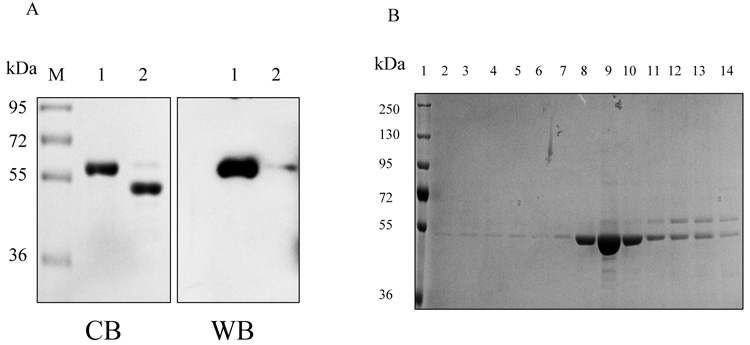
Thus, we purified the His-tagged hALT1 fusion protein into homogeneity in one step. Next, the purified recombinant proteins were pooled, dialyzed overnight, concentrated and digested with bovine EK for 24 hours at room temperature. SDS-PAGE and His-tag blotting analysis of the digested mixture showed a decrease of the high molecular weight protein and an increase of the low molecular weight protein after the EK digestion, compared to the undigested recombinant proteins, indicating the cleavage of the His-tag-containing sequence (~ 5 kDa) (Fig.3A). The protein mixture was re-charged onto a small Ni-NTA agarose column. Interestingly, cleaved ALT1 was retained on the column but was eluted with low concentration of imidazole (20 mM) in near homogeneity, but the uncut His-tag ALT1 was eluted at a higher imidazole concentration (Fig.3B). Thus, we separated cleaved hALT1 from uncut His-tag fusion protein.
Fig. 3.
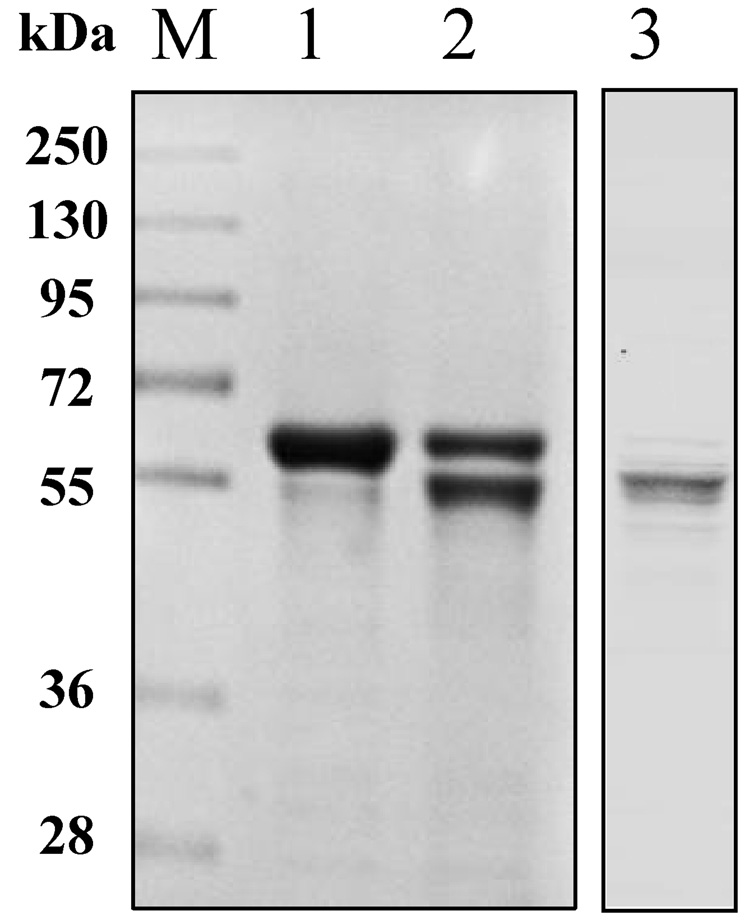
Cleavage and removal of His-tag from hALT1 and hALT2 fusion protein. (A) His-tagged ALT1 fusion proteins, undigested (lane 1) and digested (lane 2) by bovine EK, along with molecular mass marker (M), were electrophoresed for Coomassie blue (CB) staining or Western blotting (WB) with anti-His-tag antibody. (B) Coomassie blue staining of EK-digested hALT1 fractions eluted from Ni-NTA agarose column. EK-digested protein mixtures were loaded on to Ni-NTA agarose column, and step-wisely eluted with an increasing gradient of imidazole. Lane 1, molecular mass ladder; lane 2 and 3, flow-through; lane 4~6, column wash; lane7~10, 20 mM imidazole; and lane 11, 12 13 and 14 for 40, 80, 160 and 320 mM imidazole eluents, respectively. (C) Coomassie blue staining of EK-undigested (lane 1), -digested (lane 2), and His-tag free hALT2 (lane 3) eluted from Ni-NTA agarose column at 20 mM imidazole.
Recombinant hALT2 protein was purified essentially the same as the above described for ALT1 (Data not shown), except that ALT2 recombinant protein was relatively resistant to EK digestion and less stable, compared to ALT1, since a 24 hours EK digestion resulted in an incomplete digestion of the His-tagged fusion protein and some fine smaller bands appeared under the major tag-free hALT2 band (Fig. 3C). Relative enzymatic specificities of the recombinant hALT1 and hALT2 proteins during purification process were representatively summarized in Table 1. ALT activity was very low in uninfected insect cells and increased by 190 fold (from 0.04 to 7.6 U/mg) and by 10 fold (from 0.04 to 0.41 U/mg) in hALT1- and hALT2-baculovirus infected cells respectively. The lower catalytic activity in hALT2 infected cells was not due to a lower expression of hALT2 protein since the yield of purified ALT1 (6.12 mg) and ALT2 (6.0 mg) fusion protein from the corresponding crude cell lysates (ALT1: 113.8 mg; ALT2: 159 mg) was not significantly different and was likely attributed to the lower enzymatic specificity of the isoenzyme. The enzymatic activity of purified fusion or His-tag-removed ALT1 remained about 15 fold higher than that of ALT2, indicating that the specific activity of ALT1 is significantly higher than ALT2 under the current assay condition. The His-tag free ALT proteins were used for the following characterization and comparison of hALT1 and hALT2.
Table 1.
Representative yield and enzymatic activity of human ALT1 and ALT2 during purification
| Fraction | Total protein (mg) | Total activity (U) | Specific activity (U/mg protein) | |||
|---|---|---|---|---|---|---|
| hALT1 | hALT2 | hALT1 | hALT2 | hALT1 | hALT2 | |
| Uninfected cell supernatant | 0.56 | 0.56 | 0.025 | 0.025 | 0.04 | 0.04 |
| ALT lysate | 113.8 | 159 | 866.6 | 65.2 | 7.60 | 0.41 |
| His-tagged fusion protein | 6.12 | 6.0 | 329.5 | 17.6 | 53.8 | 3.0 |
| Tag-free recombinant protein | 1.38 | 0.26 | 83.1 | 1.1 | 60.2 | 4.0 |
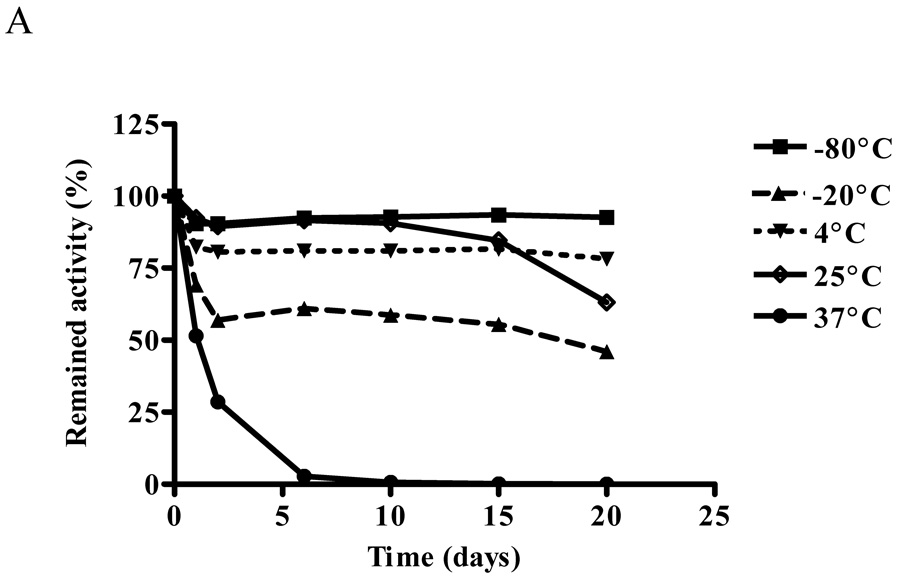
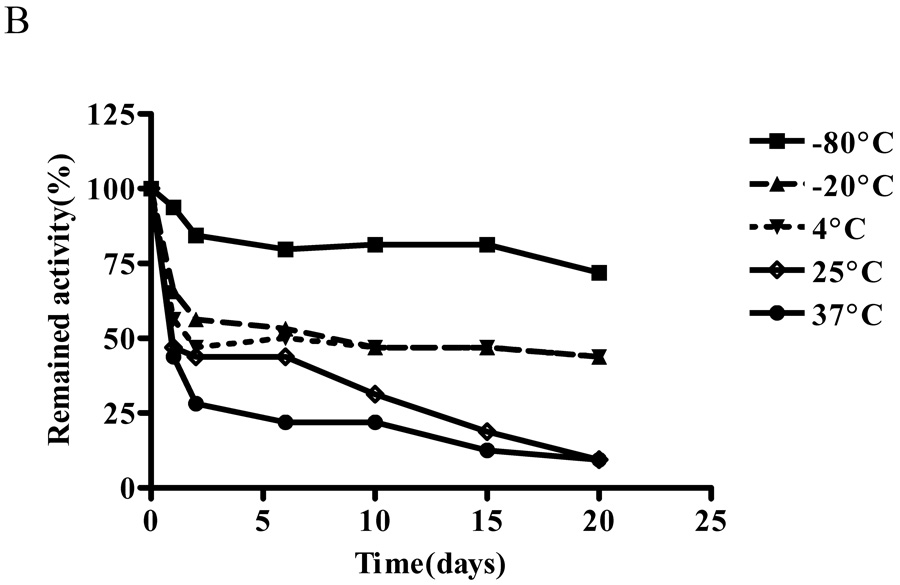
Effect of temperature and storage conditions on ALTs
The effect of temperature on ALT activity of purified recombinant human ALT proteins was studied for 20 days. In 20 mM Tris-HCl (pH 7.5) buffer at 37°C, ALT1 activity decreased rapidly and lost 50% of the activity in one day and all the activity by day 6. At 4°C, 25°C and −80°C, ALT1 activity was relative stable with 80% – 90% of the initial activity remaining through day 10, when ALT1 began to drop to 60% by day 20 at 25°C, whereas the activity remained stable at 4°C and − 80°C through day 20. Interestingly at −20°C, ALT1 lost about 43% of the activity in first 2 days, but remained relative stable thereafter till day 20 (Fig 4A). In comparison, ALT2 activity decreased quickly at all the tested temperature except −80°C (Fig 4B) and the lower the storage temperature, the better the activity was preserved. Nevertheless, the ALT2 activity lost about 72%, 56%, 53% and 44% at 37°C, 25°C, 4°C and −20°C respectively in first two days. At −80°C, ALT2 activity dropped 20% in first two days but remained stable thereafter through day 20.
Fig. 4.
Effect of temperature on enzymatic activities of recombinant hALT1 and hALT2. Purified recombinant hALT1 (1.35 µg/ml, A) or ALT2 (20 µg/ml, B) protein was added to Tris-HCl buffer (20 mM, pH7.5) and aliquoted into multiple fractions which were stored at the specified temperatures before being assayed at the indicated time points. Value is the mean of triplicate assays and expressed as the percentage of the enzyme activity at time 0.
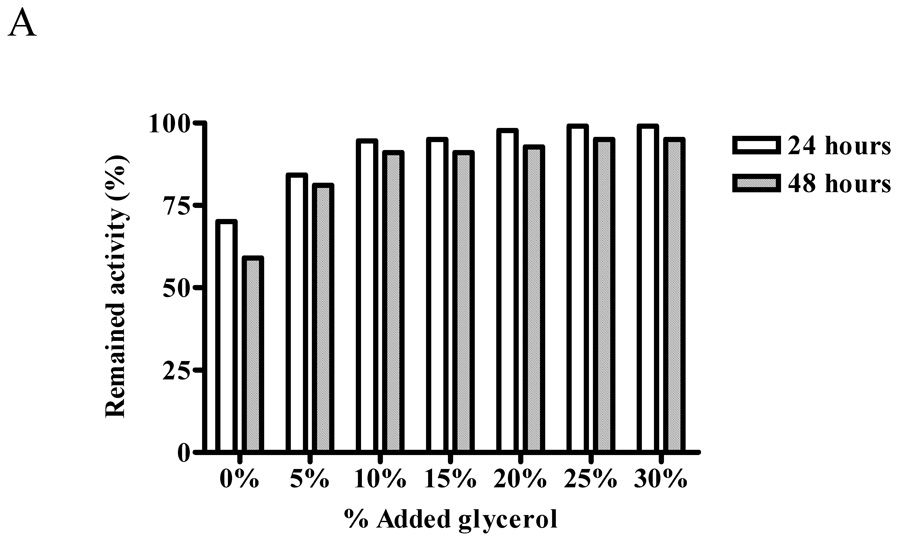
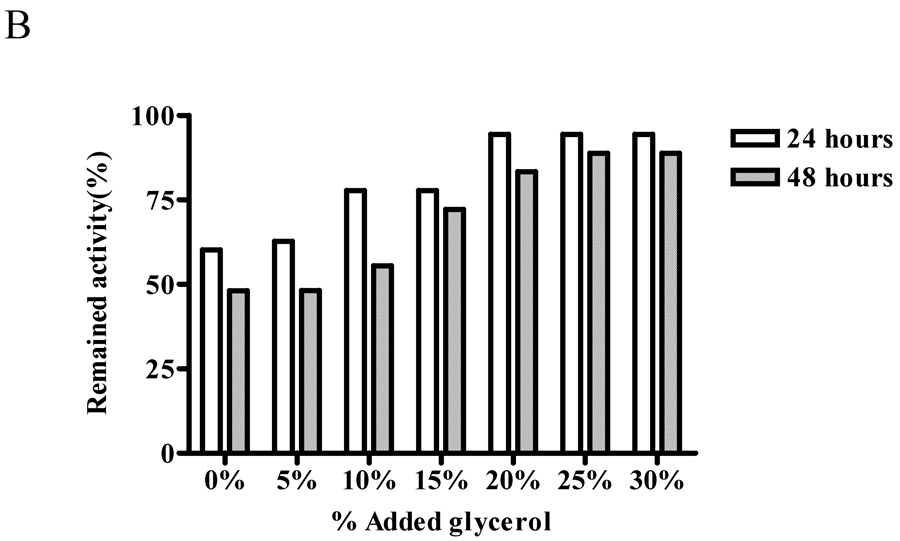
We next examined whether glycerol will help to preserve ALT activity by measuring the activity of the recombinant protein stored in various concentrations of glycerol (0–30%) in 20 mM Tris-HCl buffer, pH7.5 at −20°C for 24 and 48 hours. The preservation of ALT1 activity was noted with the increase of glycerol concentration to 25%, at which 99% and 95% of the activity remained during 24 and 48 hours storage, respectively, and further increases of glycerol concentration to 30% did not preserve more ALT1 activity (Fig. 5 panel A). In contrast, the protective effect of glycerol was more pronounced for ALT2 with increasing concentration of glycerol up to 25%, at which about 94% and 89% of the ALT2 activity was preserved for 24 and 48 hours (Fig 5 panel B). This experiment indicated that storage condition of 25% of glycerol preserved most of the ALT1 and ALT2 activity.
Fig. 5.
Protective effect of glycerol on enzymatic activities of recombinant human ALTs. Recombinant ALT1 (panel A) or ALT2 (panel B) was added to Tris-HCl buffer (20 mM, pH 7.5) containing the indicated concentrations of glycerol and stored at −20°C. Aliquots of the stored protein samples were assayed for ALT activity after storage of 24 and 48 hours. Value is the mean of triplicate assays and expressed as the percentage of the enzyme activity at time 0.
Effect of pH on ALT activity
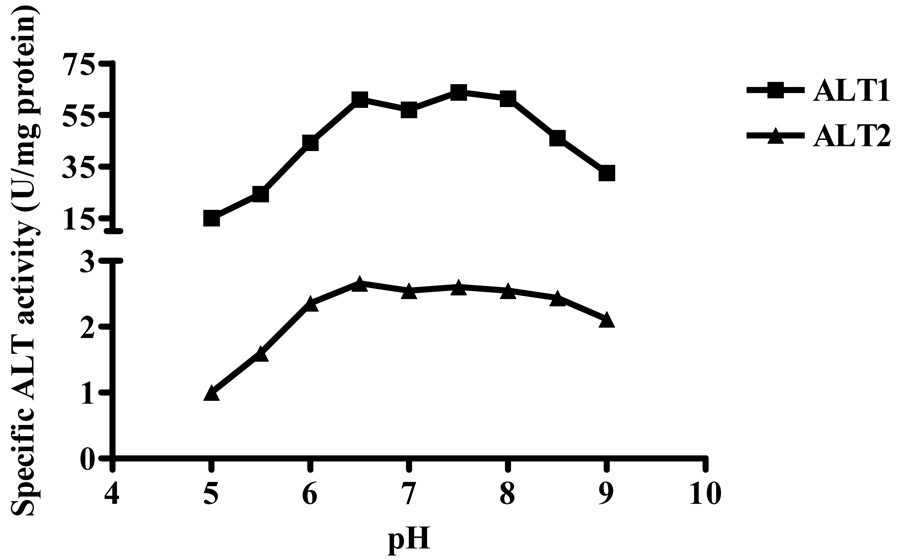
The pH profiles of the recombinant proteins were determined by measuring ALT activity at various pH values (Fig. 6). The specific activities of recombinant hALT1 increased in the range of pH 5.0 to 6.5, then flattened in the range of pH 6.5 to 8.0, but decreased from > pH 8.0 and above. For hALT2, the specific activities increased from pH 5.0 to 6.5 and then remained active over a broader pH range than hALT1 from pH 6.5 to 8.5. Hence, both hALT1 and hALT2 protein remained near fully active at pH 6.5 to 8.0.
Fig. 6.
Effect of pH on enzymatic activities of recombinant hALT1 and hALT2. Recombinant ALT1 (final 1.35 µg/ml) or ALT2 (final, 20 µg/ml) was added to Tris-HCl buffer (125 mM) at various pH values and measured for ALT activities. Value is the mean of triplicate assays and expressed as the enzyme specificity.
Discussion
More than fifty years of application of ALT activity in clinical chemistry has proven ALT as a pivotal serum marker for liver injury. But false positive or false negative signals are frequently seen in clinical practice since ALT elevation is seen in human subjects without liver damage whereas normal ALT levels are seen in patients with liver diseases. The issue of sensitivity and specificity is further compromised by variations in measuring ALT in clinical chemistry. As the current assay measures the enzyme activity rather than the enzyme protein amount, its accuracy is influenced by a number of factors, including assay temperature, substrate concentration, coenzyme concentration (e.g. pyridoxal phosphate), ionic strength of buffers, sample handing and storage conditions [11]. These factors introduce variability in serum ALT activity measurement among laboratories and make it difficult to compare data obtained from different sources [16]. Thus, a standardization of ALT assay is being recommended [11]. The discovery of ALT isoenzymes provides a new opportunity to improve the sensitivity and specificity of ALT in that these two isoenzymes are encoded by two separated genes, and differentially distributed in tissues, liver lobule and subcellular compartment (Yang et al, unpublished data). The purified hALT1 and hALT2 proteins have allowed us to conduct initial characterization of these isoforms in respect to stability and pH profile. Our studies indicate that, in a plain buffer system, both hALT1 and hALT2 are unstable at 37°C with more than 50% loss of the activity in first 24 hours, and that freezing storage at −20°C is worse than 4°C and 25°C in preserving the ALT1 activity. This result is consistent with early observations that serum ALT activity is stable for 3 days at room temperature and for 3 weeks at 4°C but decreases quickly after freezing-thawing [17, 18]. Nevertheless, we have found that the ALT activity is well preserved in 25% glycerol, a well-known stabilizer for protein activity, indicating that ALT activity may be underestimated in frozen samples and that the serum specimen should be stored at −80°C or with glycerol if the activity is to be preserved.
The purified hALT1 and hALT2 proteins showed a similar pH profile with near full activity between pH 6.5 and 8.5. This property makes it difficult to distinguish the ALT isoform activity through adjusting optimal pH for each isoform.
The specific activity of ALT1 was found to be about 15 fold higher than ALT2 during purification from crude lysate to purified protein. This finding suggests that, under current assay condition, most ALT activity measured from a tissue or serum sample would be derived from ALT1 if the level of ALT1 and ALT2 is similar. Indeed, Lindblom et al recently reported ALT1 is mainly responsible for basal ALT activity by an immunoprecipitation study [19]. This result indicates a need to develop a protein-based or activity-based ALT2 assay in order to measure the serum levels of ALT2, whose protein expression appears more restricted in the liver and muscle (Yang el al, unpublished data).
In summary, we described the expression of hALT1 and hALT2 proteins in insect cells in this report. As ALT is widely used in clinical chemistry, the recombinant proteins can be valuable to be used as a reference protein, to develop optimal assay conditions for the isoenzyme activity and to find better storage conditions to preserve the activity. Moreover, the recombinant proteins will be essential tools for the development of the protein-based and activity-based isoform-specific assay, which will facilitate our understanding of the biological and clinical significance of the ALT isoenzymes.
Acknowledgements
We thank Dr. Michael J. Betenbaugh for his advice on recombinant protein expression in High-five cell. RY is a liver scholar of the American Association for the Study of Liver Diseases. The study was partially supported by the Maryland Clinical Nutrition Research Unit Grant and the Baltimore Diabetes Research and Training Center Grant from the National Institutes of Health and a Pfizer grant.
Abbreviations used
- ALT
alanine aminotransferase
- hALT1
human ALT1
- hALT2
human ALT2
- EK
enterokinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Felig P. The glucose-alanine cycle. Metabolism. 1973;22:179–207. doi: 10.1016/0026-0495(73)90269-2. [DOI] [PubMed] [Google Scholar]
- 2.Yang RZ, Blaileanu G, Hansen BC, Shuldiner AR, Gong DW. in Genomics. 2002:445–450. doi: 10.1006/geno.2002.6722. [DOI] [PubMed] [Google Scholar]
- 3.Jadhao SB, Yang RZ, Lin Q, Hu H, Anania FA, Shuldiner AR, Gong DW. Murine alanine aminotransferase: cDNA cloning, functional expression, and differential gene regulation in mouse fatty liver. Hepatology. 2004;39:1297–1302. doi: 10.1002/hep.20182. [DOI] [PubMed] [Google Scholar]
- 4.Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB. Diagnosis and monitoring of hepatic injury. II. Recommendations for use of laboratory tests in screening, diagnosis, and monitoring [In Process Citation] Clin Chem. 2000;46:2050–2068. doi: 10.1093/clinchem/46.12.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB. Diagnosis and monitoring of hepatic injury. I. Performance characteristics of laboratory tests [In Process Citation] Clin Chem. 2000;46:2027–2049. [PubMed] [Google Scholar]
- 6.Amacher DE. Serum transaminase elevations as indicators of hepatic injury following the administration of drugs. Regul Toxicol Pharmacol. 1998;27:119–130. doi: 10.1006/rtph.1998.1201. [DOI] [PubMed] [Google Scholar]
- 7.Ruhl CE, Everhart JE. Relation of elevated serum alanine aminotransferase activity with iron and antioxidant levels in the United States. Gastroenterology. 2003;124:1821–1829. doi: 10.1016/s0016-5085(03)00395-0. [DOI] [PubMed] [Google Scholar]
- 8.Lozano M, Cid J, Bedini JL, Mazzara R, Gimenez N, Mas E, Ballesta A, Ordinas A. Study of serum alanine-aminotransferase levels in blood donors in Spain. Haematologica. 1998;83:237–239. [PubMed] [Google Scholar]
- 9.Johnston DE. Special considerations in interpreting liver function tests. Am Fam Physician. 1999;59:2223–2230. [PubMed] [Google Scholar]
- 10.Saxena S, Korula J, Shulman IA. A review of donor alanine aminotransferase testing. Implications for the blood donor and practitioner. Arch Pathol Lab Med. 1989;113:767–771. [PubMed] [Google Scholar]
- 11.Zeuzem S, Alberti A, Rosenberg W, Marcellin P, Diago M, Negro F, Prati D, Puoti C, Roberts SK, Shiffman ML. Review article: management of patients with chronic hepatitis C virus infection and "normal" alanine aminotransferase activity. Aliment Pharmacol Ther. 2006;24:1133–1149. doi: 10.1111/j.1365-2036.2006.03073.x. [DOI] [PubMed] [Google Scholar]
- 12.Kariv R, Leshno M, Beth-Or A, Strul H, Blendis L, Kokia E, Noff D, Zelber-Sagie S, Sheinberg B, Oren R, Halpern Z. Re-evaluation of serum alanine aminotransferase upper normal limit and its modulating factors in a large-scale population study. Liver Int. 2006;26:445–450. doi: 10.1111/j.1478-3231.2006.01197.x. [DOI] [PubMed] [Google Scholar]
- 13.Rajamohan F, Nelms L, Joslin DL, Lu B, Reagan WJ, Lawton M. cDNA cloning, expression, purification, distribution, and characterization of biologically active canine alanine aminotransferase-1. Protein Expr Purif. 2006;48:81–89. doi: 10.1016/j.pep.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Kost TA, Condreay JP, Jarvis DL. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat Biotechnol. 2005;23:567–575. doi: 10.1038/nbt1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cha HJ, Gotoh T, Bentley WE. Simplification of titer determination for recombinant baculovirus by green fluorescent protein marker. Biotechniques. 1997;23:782–784. 786. doi: 10.2144/97235bm03. [DOI] [PubMed] [Google Scholar]
- 16.Halfon P, Imbert-Bismut F, Messous D, Antoniotti G, Benchetrit D, Cart-Lamy P, Delaporte G, Doutheau D, Klump T, Sala M, Thibaud D, Trepo E, Thabut D, Myers RP, Poynard T. A prospective assessment of the inter-laboratory variability of biochemical markers of fibrosis (FibroTest) and activity (ActiTest) in patients with chronic liver disease. Comp Hepatol. 2002;1:3. doi: 10.1186/1476-5926-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams KM, Williams AE, Kline LM, Dodd RY. Stability of serum alanine aminotransferase activity. Transfusion. 1987;27:431–433. doi: 10.1046/j.1537-2995.1987.27587320539.x. [DOI] [PubMed] [Google Scholar]
- 18.DiMagno EP, Corle D, O'Brien JF, Masnyk IJ, Go VL, Aamodt R. Effect of long-term freezer storage, thawing, and refreezing on selected constituents of serum. Mayo Clin Proc. 1989;64:1226–1234. doi: 10.1016/s0025-6196(12)61285-3. [DOI] [PubMed] [Google Scholar]
- 19.Lindblom P, Rafter I, Copley C, Andersson U, Hedberg JJ, Berg AL, Samuelsson A, Hellmold H, Cotgreave I, Glinghammar B. Isoforms of alanine aminotransferases in human tissues and serum--differential tissue expression using novel antibodies. Arch Biochem Biophys. 2007;466:66–77. doi: 10.1016/j.abb.2007.07.023. [DOI] [PubMed] [Google Scholar]