Abstract
Estrogen is a powerful endogenous and exogenous neuroprotective agent in animal models of brain injury, including focal cerebral ischemia. Although this protection has been demonstrated in several different treatment and injury paradigms, it has not been demonstrated in focal cerebral ischemia induced by intraparenchymal endothelin-1 injection, a model with many advantages over other models of experimental focal ischemia. Reproductively mature female Sprague-Dawley rats were ovariectomized and divided into placebo and estradiol-treated groups. Two weeks later, halothane-anesthetized rats underwent middle cerebral artery (MCA) occlusion by interparenchymal stereotactic injection of the potent vasoconstrictor endothelin 1 (180 pmoles/2 µl) near the middle cerebral artery. Laser-Doppler flowmetry (LDF) revealed similar reductions in cerebral blood flow in both groups. Animals were behaviorally evaluated before, and two days after, stroke induction, and infarct size was evaluated. In agreement with other models, estrogen treatment significantly reduced infarct size evaluated by both TTC and Fluoro-Jade staining and behavioral deficits associated with stroke. Stroke size was significantly correlated with LDF in both groups, suggesting that cranial perfusion measures can enhance success in this model.
Keywords: experimental stroke, middle cerebral artery occlusion, endothelin, estrogen, neuroprotection
Introduction
Estradiol is a powerful neuroprotective agent in numerous models of cerebral injury, including experimental stroke [19, 25, 41]. In rodents, this protection has been demonstrated over wide dose ranges in males and females and in multiple models of middle cerebral artery occlusion (MCAO, for reviews see [15, 25]). The most common technique for inducing middle cerebral artery occlusion (MCAO) in the rat is through introduction of an intraluminal nylon thread through the internal carotid artery in an effort to occlude the origin of the middle cerebral artery at the circle of Willis. This method, first described by Koizumi [20], has been modified numerous times by multiple investigators in an attempt to standardize the model and reduce complications and mortality (for examples see: [1, 21, 29, 37, 45]).
In 1993, Sharkey et al. [33, 35] introduced intraparenchymal injection of the potent vasoconstrictor endothelin-1 (ET-1) as a model for focal cerebral ischemia, and demonstrated that injections very close to the MCA lead to reproducible infarcts. The advantages of the model included the relative ease of learning, minimal fine surgical skill, the ability to perform surgery in the prone position, no disturbance of the neck or mouth, low trauma and rapid recovery of subjects, and the ability to produce varying injury by altering dose. This model has been widely adopted and modified, including the use of endothelin receptor antagonists to produce reversible ischemia induced by endothelin-3 [17]. Furthermore, recent studies have demonstrated the use of this model in awake animals, mitigating the confounding effects of anesthesia [3]. In the present study, we evaluated the neuroprotective effects of estrogen in ovariectomized rats using this model.
Materials and Methods
Animals and treatments
Female Sprague-Dawley rats (7–8 weeks old; 225–250 g) were obtained from Harlan and maintained in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. The Medical College of Georgia’s Institutional Animal Care and Use Committee approved all protocols. Rats were maintained on a soy-free, reduced isoflavone custom diet (Zeigler Brothers, Inc., Gardners, PA) for one week after entering the animal facility. Rats were then ovariectomized under halothane anesthesia and randomized into one of 2 treatment groups, ovariectomy+placebo (Ovx) or ovariectomy+estradiol (Ovx+E2). Animals in the E2 group received a subcutaneous slow-release pellet of estradiol (Innovative Research, Sarasota, FL; Cat #E121, 21 day release) at the time of ovariectomy. This dose results in physiological circulating estradiol levels of approximately 25 pg/ml [22]. Two weeks later, rats underwent middle cerebral artery occlusion (MCAO) through intraparenchymal injection of endothelin-1 (described in detail below).
Middle cerebral artery occlusion
Rats were anesthetized with 5% halothane in 100% oxygen and placed in a stereotaxic frame. Animals were maintained on 1.5% halothane delivered through a nose cone. Temperature was maintained at 37±0.4 C with a servo-controlled heating pad connected to a rectal temperature probe. A midline incision was made in the scalp to expose the cranial sutures. The injection site in the left hemisphere was located using bregma as a reference point. Using a low speed hand-held drill, a small burr hole was placed in the skull at the desired location, with careful attention to avoid injuring the dura. Initial pilot studies assessed used anterior/posterior (AP) and medial/lateral (ML) coordinates similar to those used by others. However, the depth of injection was modified significantly before finding the appropriate injection location in these animals. The final coordinates used to assess estrogen-dependent neuroprotection were 0.9 mm AP, 4.8 mm ML, and 5.8 mm DV from dura. After ensuring the correct hole placement, a laser-Doppler flow probe (Perimed #403, Perimed, Stockholm, Sweden) was attached to the skull at 3 mm posterior and 5 mm lateral to bregma. After a stable flow was established, the dura was nicked with a needle and the injection pipet was lowered into place.
We constructed injection pipets for use with a pressure injection system (Toohey Pressure injection System IIE, Fairfield, NJ). A 10 mm blunt section of a 30-gauge syringe needle was cut and polished with a Dremel tool under a dissecting microscope. The end was inserted into a calibrated 5 µl glass capillary tube (World Precision Instruments, Sarasota, FL) and fixed with epoxy. After setting, the pipet was tested and washed with ethanol.
Endothelin-1 (Sigma-Aldrich, St. Louis, MO) was prepared in sterile PBS in small aliquots at 200 pmol/µl and frozen at −80C. For each experimental day a fresh aliquot of endothelin-1 was diluted in artificial CSF (CSF; in mM: 124 NaCl, 3 KCl, 1.3 MgSO4, 2.4 CaCl2, 1.4 NaH2PO4, 10 glucose, and 26 NaHCO3, pH 7.4) to provide 120 pmoles in 2 µl. The pipet was loaded and tested prior to lowering it into the brain. The pipet was lowered slowly via a sterotaxic arm and endothelin-1 was injected over 2–3 minutes using 0.5–2 msec triggers of air. Following injection, the pipet was left in place for 15 minutes before being removed. The bone was sealed with bone wax. All rats remained anesthetized with halothane for a total of 2 to 2.25 hours to monitor LDF. The scalp was closed with surgical staples after the LDF probe was removed. Rats were allowed to recover on a DeltaPhase hot pad (Braintree Scientific Inc., Braintree, MA). Sham rats were injected with 2 µl of artificial CSF.
Behavioral assessment
The day before MCAO, rats were evaluated for baseline behavior using two tests of sensory/motor function. The elevated body swing test [9] was used to assess bias in swing direction in rats elevated by the base of the tail. Twenty separate tests were performed with the number of right and left initial head/torso turns recorded. Prior to MCAO, rats swing right and left with near equal frequency, leading to a contralateral ratio of 0.5. Following MCAO, rats with cortical infarcts have a biased swing to the side contralateral to the injury with a bias approaching 1. In addition, severely injured animals develop a “c”-shaped body posture with tail elevation. The second behavioral test is the cylinder test for forepaw placement bias [31, 32]. Digital video recording of rats placed in a clear plexiglass cylinder (8” diameter × 12 “ high) is used to assess the number and order of forelimb placement on the cylinder wall during rearing. A treatment-blinded observer scored a total of the first 20 rearing wall touches to calculate an asymmetric forelimb bias with the equation: (Contralateral−Ipsilateral)/Contralateral + Ipsilateral + Both). Two days after endothelin injection, rats were tested again to assess functional deficits.
Infarct assessment
Two days after endothelin injection, and following behavioral assessment, rats were deeply anesthetized with urethane (1.5g/kg ip). Animals were then transcardially perfused with ice-cold saline. Brains were rapidly removed and placed in ice-cold sterile saline for 2 minutes. Brains were placed in a cold brain matrix (Braintree) and seven 2 mm coronal sections were made starting at the frontal pole. Sections were placed in TTC for 15 minutes at 37C and then moved to 4% formaldehyde overnight at 4C. Sections were scanned on a flatbed scanner and then frozen in OCT (Tissue Tek) for subsequent sectioning by cryostat. Digitally scanned sections were traced by hand and quantified with NIH Image. A single cryosection (10 µm) from each 2 mm slice was also processed for Fluoro-Jade B (Chemicon, Millipore, Billerica, MA) staining to determine the extent of neurodegeneration. Fluoro-Jade B sections were traced at 200x using an Olympus BX60 fluorescent microscope and Neurolucida software (MicroBrightfield Inc, Willistan, VT). Infarct size is expressed as percentage of contralateral structure with a correction for edema [36].
Statistical Analysis
Correlations between variables were assessed with Spearman Rank-Correlation Coefficient using InStat software (GraphPad, San Diego, CA). When comparing variables between Ovx and Ovx+E2, groups unpaired t-tests were used to compare single variables and analysis of variance with a posteriori Tukey-Kramer tests were used for multiple variable analysis. In all cases, a P value of <0.05 was considered significant.
Results
Ovariectomy without estrogen replacement led to a significant gain in body weight (287 ± 7 g vs 233 ± 4 g). Initial experiments were used to establish injection coordinates based on published studies. However, differences in sex, age, strain, and size made success based on published coordinates highly variable. In particular, the dorsal-ventral coordinates used by others consistently resulted in collision of the pipet with the ventral surface of the skull. Therefore, a systematic approach to determining the appropriate coordinates in our models was undertaken. Dose and concentration of endothelin, anterior-posterior injection coordinates, and LDF probe placement were consistent with other studies. However, the injection site was altered in both the medial-lateral and dorsal-ventral planes. Correlation of intended injection site and actual injection site was determined using Nissl staining. Whereas other investigators using larger male rats have used bregma to calculate DV coordinates, we found that we achieved better success using dura as our zero point.
Injection site and infarct size
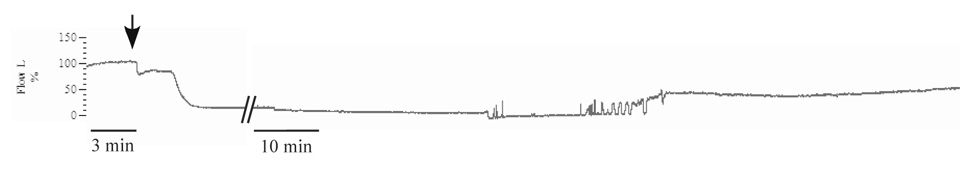
After determining the most effective coordinates for inducing stroke in young female Spague-Dawley rats, we performed regression analysis to determine which parameters are most predictive of outcome. The final coordinates used for comparison of Ovx and Ovx+E rats were 0.9 mm AP, 4.9 mm ML from Bregma, and 5.8 mm DV from dura at the injection site. The most distant injection from these coordinates was 0.8 mm dorsal. Results were analyzed for all animals and also separately for Ovx and Ovx+E animals (Table 1). Using results from 19 Ovx rats, we found that both LDF and proximity to our most effective injection site were significantly predictive of infarct site (Table 1). However, proximity to the most effective injection site did not predict the change in LDF (Table 1). In addition, although the proximity to the most effective injection site did predict infarct size overall and in Ovx rats, it did not predict infarct size in estrogen-treated rats (Table 1). Cranial LDF dropped 50–60% within 5 minutes of injection and remained reduced for at least 45 minutes, at which time recording was stopped. In 2 rats from each group, we measured cranial LDF for 3 hours. A partial reperfusion was observed beginning at about 2 hours, but did not reach preinjection levels (Fig. 1). In 3 sham rats injected with artificial CSF, a slight (<10%) drop in LDF signal was noted, and is likely due to the pressure and volume of the injection. In these animals, there was no sign of infarct after 48 hours (data not shown).
Table 1.
Spearman correlations for experimental parameters
| Group (n) | All (36) | Ovx (19) | Ovx+E (17) |
|---|---|---|---|
| LDF vs Infarct | r=0.5657 | r=0.5504 | r=0.5031 |
| P=0.0024 | P=0.0272 | P=0.0333 | |
| Site vs Infarct | r=−0.3998 | r=−0.6649 | r=−0.02158 |
| P=0.0317 | P=0.0131 | P=0.9368 | |
| Site vs LDF | r=0.01695 | r=−0.3267 | r=0.3133 |
| P=0.9279 | P=0.2346 | P=0.7457 | |
Figure 1. Representative example of cranial LDF with ET-1 injection.
LDF trace normalized to pre-injection value = 100%. Arrow represents start of ET-1 injection near the MCA. Hatches represent 60 minutes since injection and time scale is reduced for right hand portion of trace. After approximately 2 hours, several small transient increases in flow are followed by a sustained increase in LDF signal for at least an additional hour.
Effect of estrogen on infarct size
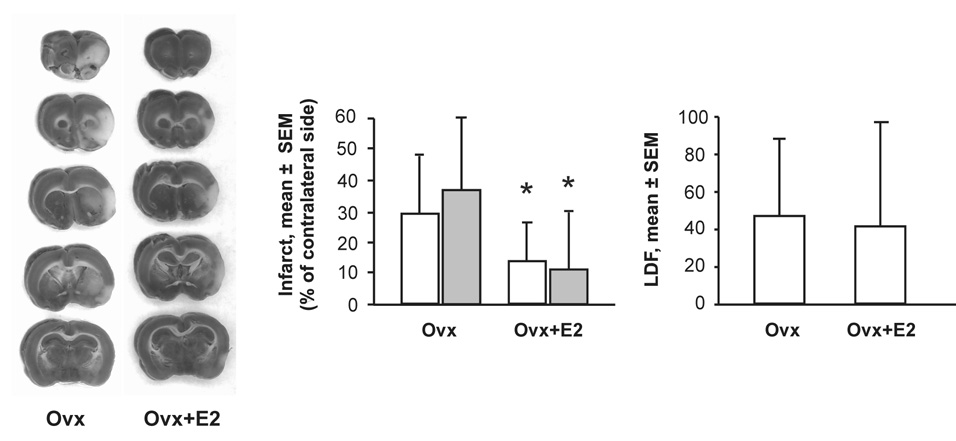
To determine the effect of estrogen on infarct size in the ET-1 stroke model, animals were randomly assigned to the Ovx or Ovx+E groups. Eight placebo and 12 estrogen treated rats completed the study. Two animals in each group died during, or shortly after, surgery. There was no significant difference between groups with respect to LDF (Fig. 2), but estrogen significantly reduced infarct size determined with TTC staining two days after induction of stroke (Fig. 2). Fluoro-Jade B staining for degenerating neurons revealed a near identical decrease in infarct size in estrogen-treated rats (Fig. 2).
Figure 2. Effect of estrogen on infarct size and cranial LDF.
Left panels show representative examples of TTC-stained brains from Ovx and Ovx+E2 rats. The unstained white area represents infarcted tissue. Left graph shows mean ± SD infarct size as a percent of the contralateral intact size in Ovx (n=8) and Ovx+E2 rats (n=12). Open bars represent calculated infarct size from TTC staining, and shaded bar represent calculated infarct size from Fluoro-Jade B staining. Right graph shows cranial LDF as percent of baseline over the first 45 minutes following injection of ET-1 in the same rats. Asterisks represent significant differences between Ovx and Ovx+ E2, P<0.05.
Effect of estrogen on behavioral response to stroke
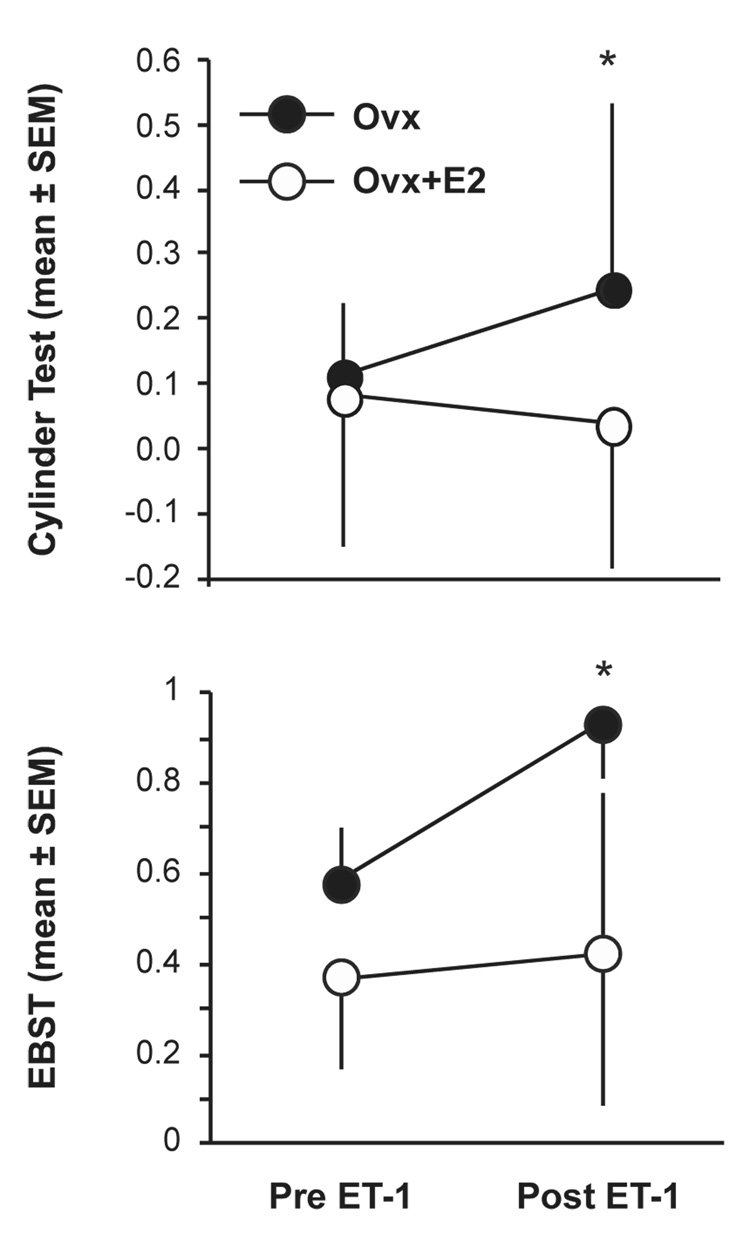
The elevated body swing test (EBST) and cylinder test were used as measures of the behavioral response to stroke. Prior to stroke, there was no significant difference between Ovx and Ovx+E rats (Fig. 3). However, 48 hours after stroke, Ovx rats displayed a significant increase in contralateral swing bias whereas estrogen-treated rats did not (Fig. 3). In the cylinder test, there was no difference between groups prior to stroke (Fig. 3). Forty-eight hours after stroke Ovx rats had a significant increase in paw placement bias, but Ovx+E rats did not (Fig. 3).
Figure 3. Effect of estrogen on behavior following ET-1 induced MCAO.
Upper panel shows mean ± SD of ipsilateral paw preference in the cylinder test in Ovx (n=8) and Ovx+E2 rats (n=12) 48 hours after stroke. Lower graph shows mean ± SD of contralateral swing bias in the EBST in Ovx (n=8) and Ovx+E2 rats (n=12) 48 hours after stroke. Asterisks represent significant differences, P<0.05.
Discussion
Estrogen is neuroprotective in multiple models of focal and global ischemia. However, this effect has not been previously demonstrated in female rats using intraparechymal endothelin injection in the vicinity of the middle cerebral artery. Compared with the intraluminal thread model, adaptation of this technique to the young female Spague-Dawley rat provides an additional experimental model that is less technically difficult, less invasive, and provides the options of performing other stereotaxic procedures during stroke. The consistent correlation of LDF with infarct size further supports the use of LDF monitoring in focal ischemic models. Furthermore, the present study provides additional support for the growing list of neuroprotective actions of estrogen, as both infarct volume and behavioral deficits were mitigated in estrogen-treated rats.
Although the ET-1 stroke model has gained wide acceptance, its application to female rats has been limited to two reports [42, 43]. In addition, few investigators have used real-time measures of cerebral blood flow to confirm effectiveness of injections [6, 17]. A recent systematic review of methods used to produce ischemia with ET-1 showed only a 50% success rate in producing rats that survive at least one week and display behavioral deficits when MCAO was accomplished through parenchymal injection of ET-1 near the MCA [40]. In the present study, we found a significant correlation between LDF drop and infarct size, suggesting that success in this model might be improved by LDF measurements during surgery. Most recent studies using ET-1-induced strokes do not evaluate cerebral blood flow. The low success rate demonstrated by Windle [40] has the potential to significantly impact not only the use of the model for producing stroke, but also the ability to evaluate the effects of neuroprotective agents if LDF is not equilibrated among experimental and control groups. In the present study, we demonstrated a significant correlation between cranial LDF and infarct size, and this relationship was maintained in both the OVX and OVX+E groups. Similarly, Biernaskie [6] showed a high correlation between 8 hour cerebral perfusion determined with MRI and histological outcome at 7 days.
Injection of ET-1 near the MCA has been used by several investigators to demonstrate therapeutic approaches to stroke, including neuroprotection via immune suppression [34], sodium channel blockade [10], calcium channel blockade [7, 28], and NMDA receptor blockade [26]. Using direct aplication of ET-1 to the MCA through craniotomy Dawson, et al. also demonstrated the protective effects of free radical scavengers [12]. Preconditioning effects of thrombin are seen using ET-1-induced MCAO [16], and prophylactic neuroprotective effects of growth factors have also been observed [4]. In contrast, a metabotropic glutamate receptor agonist that is effective in reducing global ischemic damage in gerbils did not have neuroprotective effects in rats injected with ET-1 [8]. Studies using interparenchymal injections of ET-1 into the cortex or striatum have also demonstrated neuroprotective effects of minocyline [18] and nitric oxide donors [23].
One potential caveat of the present study, and all studies using ET-1 injection, is the potential interaction of the neuroprotective agent with ET-1 signaling pathways, including ET-A and ET-B receptors. Limited in vitro data suggest that estrogens can reduce ET-1-dependent vascular tone in the aorta [2] and cerebral vasculature [39]. Estrogen can also reduce ET-1 expression in the aorta [38]. However, the effects of ET-1 injection are likely to be more dependent on estrogen regulation of ET receptors, for which little is known. In the rat heart, estrogen increases ET-B, but not ET-A receptor expression [27]. Similarly, estrogen increases ET-B receptor mRNA in the trigeminal ganglion [30]. However, measurement of cranial LDF at a site distal to injection of ET-1 further supports the use of this model. The observation that cranial LDF was not different between Ovx and Ovx+E rats, although infarct size was, suggests that any effects of estrogens on the ET-1 response are not mediated by early changes in flow.
Another potential concern with the ET-1 MCAO model is how it can best be compared with other models of stroke, particularly the intraluminal thread model. Does ET-1 injection near the MCA represent a permanent or transient occlusion? In our hands, flow remains significantly reduced up to two hours. This is followed by a partial reperfusion that is maintained for at least another 3 hours. Similarly, Gartshore et al. demonstrated limited reperfusion of the cortex starting two hours after ET-1 application to the MCA through craniotomy [14]. Biernaskie also demonstrated limited reperfusion 7–48 hours after ET-1 injection [6], proposing that the long-lasting moderate reduction in blood flow followed by slow, but near complete reperfusion may more accurately reflect human stroke [6]. For more direct comparison with intraluminal thread models of controlled reperfusion, more rapid and complete reperfusion is possible with ET-3 and ET-R antagonist injections [17], although we have not explored this method. The injury induced by ET-1 injection produces both a localized vasospasm close to the injection site and reduced perfusion in distal portions of the vascular tree. The vasospasm itself may retain characteristics associated with responses to subarachnoid hemorrhage which are themselves partially mediated by ET-1 [11] Endothelin increases in the brain following both focal and global ischemia and likely contributes to ischemic injury [5, 44] because blockade of central ET-A receptors is itself neuroprotective in transient [24] and permanent MCAO [13]. Thus this model of brain injury likely retains properties associated both with embolic stroke and other forms of ischemic injury.
In conclusion, focal ischemia produced by intraparenchymal injection of ET-1 close to the MCA provides a suitable model for the examination of estrogen’s neuroprotective effects. However, careful monitoring of cranial perfusion and consistent exclusion criteria are necessary to ensure that the extent of ischemia is the same across treatment groups.
Acknowledgements
This work was supported by NIH R01 AT001882 to D.A.S. The authors thank Drs. Ann Schreihofer and Adviye Ergul for helpful comments on this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature References
- 1.Aspey BS, Cohen S, Patel Y, Terruli M, Harrison MJ. Middle cerebral artery occlusion in the rat: consistent protocol for a model of stroke. Neuropathol Appl Neurobiol. 1998;24:487–497. doi: 10.1046/j.1365-2990.1998.00146.x. [DOI] [PubMed] [Google Scholar]
- 2.Ba ZF, Lu A, Shimizu T, Szalay L, Schwacha MG, Rue LW, 3rd, Bland KI, Chaudry IH. 17beta-Estradiol modulates vasoconstriction induced by endothelin-1 following trauma-hemorrhage. Am J Physiol Heart Circ Physiol. 2007;292:H245–H250. doi: 10.1152/ajpheart.00809.2006. [DOI] [PubMed] [Google Scholar]
- 3.Baldauf K, Henrich-Noack P, Reymann KG. Detrimental effects of halothane narcosis on damage after endothelin-1-induced MCAO. J Neurosci Methods. 2006;162:14–18. doi: 10.1016/j.jneumeth.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Baldauf K, Reymann KG. Influence of EGF/bFGF treatment on proliferation, early neurogenesis and infarct volume after transient focal ischemia. Brain Res. 2005;1056:158–167. doi: 10.1016/j.brainres.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 5.Barone FC, Globus MY, Price WJ, White RF, Storer BL, Feuerstein GZ, Busto R, Ohlstein EH. Endothelin levels increase in rat focal and global ischemia. J Cereb Blood Flow Metab. 1994;14:337–342. doi: 10.1038/jcbfm.1994.41. [DOI] [PubMed] [Google Scholar]
- 6.Biernaskie J, Corbett D, Peeling J, Wells J, Lei H. A serial MR study of cerebral blood flow changes and lesion development following endothelin-1-induced ischemia in rats. Magn Reson Med. 2001;46:827–830. doi: 10.1002/mrm.1263. [DOI] [PubMed] [Google Scholar]
- 7.Bogaert L, O'Neill MJ, Moonen J, Sarre S, Smolders I, Ebinger G, Michotte Y. The effects of LY393613, nimodipine and verapamil, in focal cerebral ischaemia. Eur J Pharmacol. 2001;411:71–83. doi: 10.1016/s0014-2999(00)00861-x. [DOI] [PubMed] [Google Scholar]
- 8.Bond A, Ragumoorthy N, Monn JA, Hicks CA, Ward MA, Lodge D, O'Neill MJ. LY379268, a potent and selective Group II metabotropic glutamate receptor agonist, is neuroprotective in gerbil global, but not focal, cerebral ischaemia. Neurosci Lett. 1999;273:191–194. doi: 10.1016/s0304-3940(99)00663-1. [DOI] [PubMed] [Google Scholar]
- 9.Borlongan CV, Sanberg PR. Elevated body swing test: a new behavioral parameter for rats with 6-hydroxydopamine-induced hemiparkinsonism. J Neurosci. 1995;15:5372–5378. doi: 10.1523/JNEUROSCI.15-07-05372.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callaway JK, Castillo-Melendez M, Giardina SF, Krstew EK, Beart PM, Jarrott B. Sodium channel blocking activity of AM-36 and sipatrigine (BW619C89): in vitro and in vivo evidence. Neuropharmacology. 2004;47:146–155. doi: 10.1016/j.neuropharm.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Crowley RW, Medel R, Kassell NF, Dumont AS. New insights into the causes and therapy of cerebral vasospasm following subarachnoid hemorrhage. Drug Discov Today. 2008;13:254–260. doi: 10.1016/j.drudis.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Dawson DA, Masayasu H, Graham DI, Macrae IM. The neuroprotective efficacy of ebselen (a glutathione peroxidase mimic) on brain damage induced by transient focal cerebral ischaemia in the rat. Neurosci Lett. 1995;185:65–69. doi: 10.1016/0304-3940(94)11226-9. [DOI] [PubMed] [Google Scholar]
- 13.Dawson DA, Sugano H, McCarron RM, Hallenbeck JM, Spatz M. Endothelin receptor antagonist preserves microvascular perfusion and reduces ischemic brain damage following permanent focal ischemia. Neurochem Res. 1999;24:1499–1505. doi: 10.1023/a:1021139713026. [DOI] [PubMed] [Google Scholar]
- 14.Gartshore G, Dawson D, Patterson J, Macrae IM. Topographic profile of reperfusion into MCA territory following endothelin-1-induced transient focal cerebral ischaemia. Neurosci Lett. 1996;202:209–213. doi: 10.1016/0304-3940(95)12236-2. [DOI] [PubMed] [Google Scholar]
- 15.Gibson CL, Gray LJ, Murphy SP, Bath PM. Estrogens and experimental ischemic stroke: a systematic review. J Cereb Blood Flow Metab. 2006;26:1103–1113. doi: 10.1038/sj.jcbfm.9600270. [DOI] [PubMed] [Google Scholar]
- 16.Henrich-Noack P, Striggow F, Reiser G, Reymann KG. Preconditioning with thrombin can be protective or worsen damage after endothelin-1-induced focal ischemia in rats. J Neurosci Res. 2006;83:469–475. doi: 10.1002/jnr.20746. [DOI] [PubMed] [Google Scholar]
- 17.Henshall DC, Butcher SP, Sharkey J. A rat model of endothelin-3-induced middle cerebral artery occlusion with controlled reperfusion. Brain Res. 1999;843:105–111. doi: 10.1016/s0006-8993(99)01896-x. [DOI] [PubMed] [Google Scholar]
- 18.Hewlett KA, Corbett D. Delayed minocycline treatment reduces long-term functional deficits and histological injury in a rodent model of focal ischemia. Neuroscience. 2006;141:27–33. doi: 10.1016/j.neuroscience.2006.03.071. [DOI] [PubMed] [Google Scholar]
- 19.Hurn PD, Brass LM. Estrogen and stroke: a balanced analysis. Stroke. 2003;34:338–341. doi: 10.1161/01.str.0000054051.88378.25. [DOI] [PubMed] [Google Scholar]
- 20.Koizumi JYY, Nakazawa T. Ooneda G., Experimental studies of ischemic brain edema, I: a new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Jpn J Stroke. 1986;8:1–8. [Google Scholar]
- 21.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 22.Lovekamp-Swan T, Glendenning ML, Schreihofer DA. A high soy diet enhances neurotropin receptor and Bcl-XL gene expression in the brains of ovariectomized female rats. Brain Res. 2007;1159:54–66. doi: 10.1016/j.brainres.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez-Murillo R, Fernandez AP, Serrano J, Rodrigo J, Salas E, Mourelle M, Martinez A. The nitric oxide donor LA 419 decreases brain damage in a focal ischemia model. Neurosci Lett. 2007;415:149–153. doi: 10.1016/j.neulet.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Matsuo Y, Mihara S, Ninomiya M, Fujimoto M. Protective effect of endothelin type A receptor antagonist on brain edema and injury after transient middle cerebral artery occlusion in rats. Stroke. 2001;32:2143–2148. doi: 10.1161/hs0901.94259. [DOI] [PubMed] [Google Scholar]
- 25.McCullough LD, Hurn PD. Estrogen and ischemic neuroprotection: an integrated view. Trends Endocrinol Metab. 2003;14:228–235. doi: 10.1016/s1043-2760(03)00076-6. [DOI] [PubMed] [Google Scholar]
- 26.Moyanova SG, Kortenska LV, Mitreva RG, Pashova VD, Ngomba RT, Nicoletti F. Multimodal assessment of neuroprotection applied to the use of MK-801 in the endothelin-1 model of transient focal brain ischemia. Brain Res. 2007;1153:58–67. doi: 10.1016/j.brainres.2007.03.070. [DOI] [PubMed] [Google Scholar]
- 27.Nuedling S, van Eickels M, Allera A, Doevendans P, Meyer R, Vetter H, Grohe C. 17 Beta-estradiol regulates the expression of endothelin receptor type B in the heart. Br J Pharmacol. 2003;140:195–201. doi: 10.1038/sj.bjp.0705409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Neill MJ, Hicks CA, Ward MA, Osborne DJ, Wishart G, Mathews KS, McLaughlin DP, Stamford JA, McCarty DR, Patrick KE, Roman C, Fleisch JH, Gilmore J, Boot JR. LY393615, a novel neuronal Ca(2+) and Na(+) channel blocker with neuroprotective effects in models of in vitro and in vivo cerebral ischemia. Brain Res. 2001;888:138–149. doi: 10.1016/s0006-8993(00)03043-2. [DOI] [PubMed] [Google Scholar]
- 29.Oliff HS, Weber E, Eilon G, Marek P. The role of strain/vendor differences on the outcome of focal ischemia induced by intraluminal middle cerebral artery occlusion in the rat. Brain Res. 1995;675:20–26. doi: 10.1016/0006-8993(95)00033-m. [DOI] [PubMed] [Google Scholar]
- 30.Puri V, Puri S, Svojanovsky SR, Mathur S, Macgregor RR, Klein RM, Welch KM, Berman NE. Effects of oestrogen on trigeminal ganglia in culture: implications for hormonal effects on migraine. Cephalalgia. 2006;26:33–42. doi: 10.1111/j.1468-2982.2005.00987.x. [DOI] [PubMed] [Google Scholar]
- 31.Roof RL, Schielke GP, Ren X, Hall ED. A comparison of long-term functional outcome after 2 middle cerebral artery occlusion models in rats. Stroke. 2001;32:2648–2657. doi: 10.1161/hs1101.097397. [DOI] [PubMed] [Google Scholar]
- 32.Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 33.Sharkey J, Butcher SP. Characterisation of an experimental model of stroke produced by intracerebral microinjection of endothelin-1 adjacent to the rat middle cerebral artery. J Neurosci Methods. 1995;60:125–131. doi: 10.1016/0165-0270(95)00003-d. [DOI] [PubMed] [Google Scholar]
- 34.Sharkey J, Crawford JH, Butcher SP, Marston HM. Tacrolimus (FK506) ameliorates skilled motor deficits produced by middle cerebral artery occlusion in rats. Stroke. 1996;27:2282–2286. doi: 10.1161/01.str.27.12.2282. [DOI] [PubMed] [Google Scholar]
- 35.Sharkey J, Ritchie IM, Kelly PA. Perivascular microapplication of endothelin-1: a new model of focal cerebral ischaemia in the rat. J Cereb Blood Flow Metab. 1993;13:865–871. doi: 10.1038/jcbfm.1993.108. [DOI] [PubMed] [Google Scholar]
- 36.Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 37.Takano K, Tatlisumak T, Bergmann AG, Gibson DG, 3rd, Fisher M. Reproducibility and reliability of middle cerebral artery occlusion using a silicone-coated suture (Koizumi) in rats. J Neurol Sci. 1997;153:8–11. doi: 10.1016/s0022-510x(97)00184-6. [DOI] [PubMed] [Google Scholar]
- 38.Tan Z, Wang TH, Yang D, Fu XD, Pan JY. Mechanisms of 17beta-estradiol on the production of ET-1 in ovariectomized rats. Life Sci. 2003;73:2665–2674. doi: 10.1016/s0024-3205(03)00605-2. [DOI] [PubMed] [Google Scholar]
- 39.Tsang SY, Yao X, Chan FL, Wong CM, Chen ZY, Laher I, Huang Y. Estrogen and tamoxifen modulate cerebrovascular tone in ovariectomized female rats. Hypertension. 2004;44:78–82. doi: 10.1161/01.HYP.0000131659.27081.19. [DOI] [PubMed] [Google Scholar]
- 40.Windle V, Szymanska A, Granter-Button S, White C, Buist R, Peeling J, Corbett D. An analysis of four different methods of producing focal cerebral ischemia with endothelin-1 in the rat. Exp Neurol. 2006;201:324–334. doi: 10.1016/j.expneurol.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 41.Wise PM, Dubal DB, Wilson ME, Rau SW. Estradiol is a neuroprotective factor in in vivo and in vitro models of brain injury. J Neurocytol. 2000;29:401–410. doi: 10.1023/a:1007169408561. [DOI] [PubMed] [Google Scholar]
- 42.Yager JY, Wright S, Armstrong EA, Jahraus CM, Saucier DM. A new model for determining the influence of age and sex on functional recovery following hypoxic-ischemic brain damage. Dev Neurosci. 2005;27:112–120. doi: 10.1159/000085982. [DOI] [PubMed] [Google Scholar]
- 43.Yager JY, Wright S, Armstrong EA, Jahraus CM, Saucier DM. The influence of aging on recovery following ischemic brain damage. Behav Brain Res. 2006;173:171–180. doi: 10.1016/j.bbr.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 44.Yamashita K, Kataoka Y, Niwa M, Shigematsu K, Himeno A, Koizumi S, Taniyama K. Increased production of endothelins in the hippocampus of stroke-prone spontaneously hypertensive rats following transient forebrain ischemia: histochemical evidence. Cell Mol Neurobiol. 1993;13:15–23. doi: 10.1007/BF00712986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang Y, Yang T, Li Q, Wang CX, Shuaib A. A new reproducible focal cerebral ischemia model by introduction of polyvinylsiloxane into the middle cerebral artery: a comparison study. J Neurosci Methods. 2002;118:199–206. doi: 10.1016/s0165-0270(02)00142-5. [DOI] [PubMed] [Google Scholar]