Abstract
Context
The “de novo” formation of fluid-conducting patterns by tumor cells, termed vasculogenic mimicry (VM), is associated with increased mortality in many different solid tumors.
Objective
To identify VM patterns in hepatocellular carcinoma (HCC) and to determine whether these patterns were associated with more rapid tumor recurrence after orthotopic liver transplantation.
Design
Subjects included 20 patients who underwent orthotopic liver transplantation and were found to have HCC in the liver explant. Samples from 5 normal postmortem livers and 5 explanted livers with hepatitis C virus cirrhosis without HCC served as control tissues. Patterned matrix VM expression in HCC was identified by the presence of laminin-positive loops surrounding packets of tumor cells. Time to HCC recurrence after orthotopic liver transplantation was compared between patients with and without patterned VM expression. The relationships among VM in HCC, cause of chronic liver disease, serum α-fetoprotein level at the time of diagnosis, tissue expression by epidermal growth factor receptor, and endothelial markers including vascular endothelial growth factor and CD31 were assessed.
Results
Patterned matrix VM was identified in 11 of 20 primary HCC tissue samples. Vasculogenic mimicry was absent in all 10 control cases and was not identified in any area of dysplasia. The expression of VM in HCC lesions in liver explants was associated with more rapid posttransplant recurrence (P = .01). Vasculogenic mimicry was not associated with the cause of liver disease, serum α-fetoprotein level at time of diagnosis, or expression of epidermal growth factor receptor, vascular endothelial growth factor, or CD31.
Conclusions
Vasculogenic mimicry of the patterned matrix type is present in hepatocellular carcinoma and is associated with tumor recurrence after orthotopic liver transplantation. Vasculogenic mimicry lesions are not associated with endothelial markers in HCC.
Vasculogenic mimicry (VM)—the development of fluid-conducting pathways by highly invasive and genetically dysregulated tumor cells—appears in 2 forms. In VM of the tubular type, non–endothelial cell-lined tubes resembling blood vessels are identified. In VM of the patterned matrix type, sheaths of extracellular matrix rich in laminin, collagens IV and VI, fibronectin, and heparan sulfate proteoglycan form loops surrounding packets of tumor cells.1 The extracellular matrix connects to endothelial cell lined blood vessels and transmits fluid, forming a fluid-conducting meshwork.2 Vasculogenic mimicry has been described in many tumors including melanoma,1,3–6 inflammatory and ductal breast carcinoma,7 ovarian carcinoma,8,9 prostatic carcinoma,10,11 synovial sarcoma,12,13 rhabdomyosarcoma,12,13 osteosarcoma,14 and pituitary tumors.15 The presence of VM has been associated with more aggressive tumor biology and increased tumor-related mortality.4,16,17
There are limited data regarding VM in hepatocellular carcinoma (HCC). Tumor “vascularity” was partly attributed to VM in a transgenic mouse model of HCC.18 Two studies described the presence of “vasculogenic mimicry” in human HCC,19,20 although the endothelial cell markers used identified the tubular type but do not detect patterned matrix VM.1 Consequently, patterned matrix VM has not been systematically evaluated in human HCC lesions. This pilot study was designed to determine whether VM of the patterned matrix type occurs in HCC, and if so, to evaluate for an association between VM and HCC recurrence after orthotopic liver transplantation (OLT).
MATERIALS AND METHODS
Tissue blocks from 20 consecutive patients who underwent OLT for HCC were studied. Tissue specimens from 5 normal livers and 5 patients with hepatitis C–related cirrhosis served as controls. The study protocol was approved by the institutional review board at the University of Illinois at Chicago (UIC).
Evaluation of Tumor Tissue in Liver Explants
Liver explants were sectioned horizontally at 1-cm intervals. A histologic section was obtained from cirrhotic tissue and any nodules that differed in size or color from the cirrhotic liver tissue. The physical characteristics of the tumor including size, location, appearance, and number were recorded. Hematoxylin-eosin–stained slides were reviewed for evaluation of tumor type, grade, and stage following the guidelines of the World Health Organization (WHO) and International Union Against Cancer (UICC).21 Areas of dysplasia were characterized as groups of cells containing nuclei with abnormal sizes, inconsistent configurations, and occasional multinucleation in regions with partial or complete nodular replacement and normal liver cell plate thickness.22,23
Immunohistochemical Studies
Serial 4-μm sections were obtained from formalin-fixed and paraffin-embedded tumor tissue. Hematoxylin-eosin staining was performed to evaluate histologic features of HCC. Slides were examined for VM by 3 independent observers who were blinded to outcome. Laminin staining was used as the primary indicator of VM.1,4 Vasculogenic mimicry patterns were identified by the detection of laminin-positive loops surrounding clusters of 3 to 15 tumor cells. Red blood cells were variably present within these patterns. There was agreement among observers in all cases. Hematoxylin-eosin–stained slides were reviewed for confirmation of tumor type, grade, and stage.24 Standard immunohistochemical staining was performed on paraffin-embedded tumor blocks and control tissues for laminin, epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF), and CD31.
Procedure for Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue samples were sectioned at 4 μm thickness and mounted on Superfrost/Plus slides. Slides were deparaffinized in xylene and rehydrated through a decreasing ethanol gradient. Slides were rinsed in distilled water followed by antigen unmasking utilizing a 10× concentrated retrieval solution (Target Retrieval Solution, Dako, Carpinteria, Calif) according to manufacturer’s instructions and then rinsed in phosphate-buffered saline for 5 minutes. For the demonstration of laminin, EGFR, VEGF, and CD31, blocking solution (Peroxidase Blocking Reagent, Dako) was applied for 10 minutes at room temperature. Slides were pretreated with proteinase K (Dako) for 5 minutes. Slides were treated with protein blocking solution (Protein Block Serum-Free, Dako) for 10 minutes at room temperature, then rinsed and incubated with monoclonal mouse anti-laminin antibody (L8271, clone LAM 89, Sigma Aldrich, St Louis, Mo) at a titer of 1:200; mouse anti-EGFR (clone 2-18C9, Dako) at a titer of 1:200; anti-human VEGF antibody (555036, BD Pharmingen, San Diego, Calif) at a titer of 1:50; or monoclonal mouse anti-human CD31 antibody (M0823, Dako) at a titer of 1:25; for 30 minutes at room temperature. Slides were rinsed, then treated with EnVision+-labeled polymer (Dako) for 30 minutes at room temperature. Immunohistochemical staining for the different antibodies was detected by DAB+ (Dako) for 10 minutes. Slides were rinsed in distilled water, counterstained, and dehydrated through an alcohol gradient and mounted with Permount.
EGFR or VEGF Staining Intensity Index
Membranous staining for EGFR and cytoplasmic staining for VEGF and CD31 were considered positive. The intensity of staining for EGFR and VEGF was graded as 0 for absent immunoreactivity, 1 for weak, 2 for moderate, and 3 for intense positivity. The percentage of positivity within the tumor was assessed. A staining intensity index was calculated by multiplying the percentage of the entire tumor sample staining positively and the intensity grade (eg, 80 × 2 = staining intensity index of 160).25,26
Surveillance for HCC Recurrence After Liver Transplantation
Liver transplant recipients underwent cross-sectional imaging on a 3- to 6-month basis to assess for evidence of posttransplant recurrence. Time to tumor recurrence was based on the interval from transplantation to detection of characteristic features of HCC on contrast enhanced computerized tomography or magnetic resonance imaging.
Statistical Analysis
Normally distributed variables were compared between patients with and without VM expression using a Student t test, and categorical variables were compared using the Pearson χ2 test or Fisher exact test when appropriate. Time to HCC recurrence was estimated by the Kaplan-Meier product-limit method. Survival distributions were compared between patients with and without VM by univariate analysis using the log-rank test. Multivariate analysis was then performed using the Cox proportional hazards model. Only 2 variables were entered into the multivariate model at a time due to the small sample size. Statistical analysis was performed using SPSS (version 12.0, Chicago, Ill).
RESULTS
VM in HCC and Control Specimens
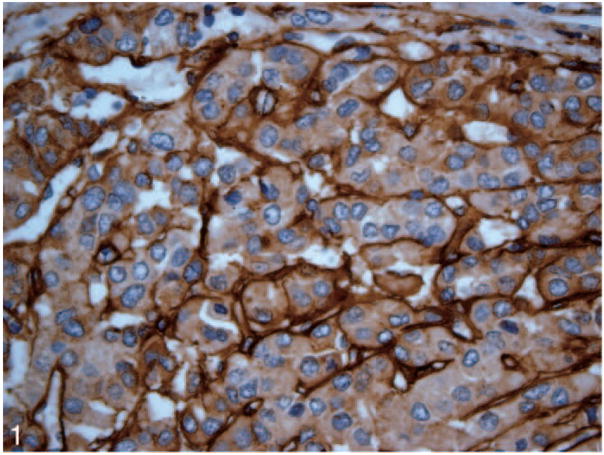
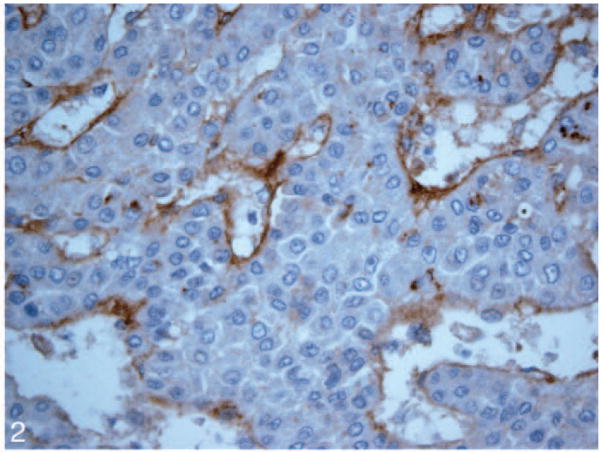
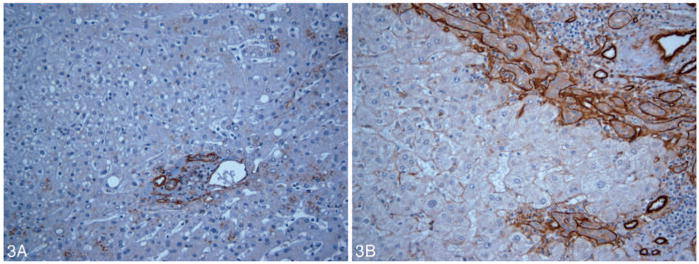
Patterned matrix vasculogenic mimicry was identified in HCC tissue from 11 (55%) of 20 liver explants including 8 of 9 cases that subsequently recurred posttransplant (Figure 1). Nine primary HCC lesions showed no evidence of VM (Figure 2). None of the control tissues including specimens from 5 normal livers and samples from 5 cirrhotic livers without HCC (Figure 3, A and B) contained VM.
Figure 1.
Vasculogenic mimicry expression is identified in a hepatocellular carcinoma lesion by laminin immunostain (original magnification ×40).
Figure 2.
A hepatocellular carcinoma lesion without vasculogenic mimicry expression by laminin immunostain (original magnification ×40).
Figure 3.
A, Vasculogenic mimicry is absent in nonneoplastic hepatocytes stained by laminin (original magnification ×20). Normal portal bile duct and vascular basement membranes are positive for laminin. B, Vasculogenic mimicry expression is absent in hepatocytes in a section of an explanted cirrhotic liver stained by laminin (original magnification ×20).
Comparison of VM-Positive and VM-Negative HCC Cases
There was no difference in demographic features including sex, age, or cause of liver disease (viral or other) between subjects with VM-positive and VM-negative tumors (Tables 1 and 2). The tumor size, grade, stage, and presence of lymphovascular space tumor invasion did not distinguish VM-positive and VM-negative cases (Table 1). The α-fetoprotein serum level (20 ng/mL [P = .10]) at the time of diagnosis did not correlate with the presence of VM.
Table 1.
Comparison of Clinical Factors as a Function of Vasculogenic Mimicry (VM) Expression in Hepatocellular Carcinoma
| VM Positive (n = 11) | VM Negative (n = 9) | P Value | |
|---|---|---|---|
| Sex (F/M) | 5/6 | 2/7 | .27 |
| Age, mean ± SD, y | 52 ± 13 | 57 ± 13 | .95 |
| Cause of liver disease (viral/other) | 9/2 | 5/4 | .34 |
| Tumor size (≤5 cm/>5 cm) | 8/3 | 7/2 | .43 |
| Tumor grade (low [1]/high [2 or 3]) | 3/8 | 1/8 | .38 |
| Tumor stage (low [1]/high [2 or 3]) | 8/3 | 4/5 | .65 |
| Lymphovascular space tumor invasion (−/+) | 9/2 | 9/0 | .29 |
Table 2.
Comparison of Cause of Liver Disease and Vasculogenic Mimicry (VM) Expression and Tumor Recurrence
| Cause of Liver Disease | VM Positive | VM Negative | Tumor Recurrence |
|---|---|---|---|
| Autoimmune hepatitis (n = 1) | 0 | 1 | 0 |
| Cryptogenic cirrhosis (n = 1) | 1 | 0 | 1 |
| Alcohol-induced cirrhosis (n = 1) | 1 | 0 | 1 |
| Chronic hepatitis B virus cirrhosis (n = 1) | 1 | 0 | 1 |
| Chronic hepatitis C virus cirrhosis (n = 12) | 7 | 5 | 6* |
| Chronic hepatitis C virus and alcohol-induced cirrhosis (n = 1) | 1 | 0 | 0 |
| Nonalcoholic steatohepatitis (n = 2) | 0 | 2 | 0 |
| Primary biliary cirrhosis (n = 1) | 0 | 1 | 0 |
| Total | 11 | 9 | 9 |
Recurrences were positive for VM in 5 of 6 cases.
VM Expression and Time to Recurrent HCC
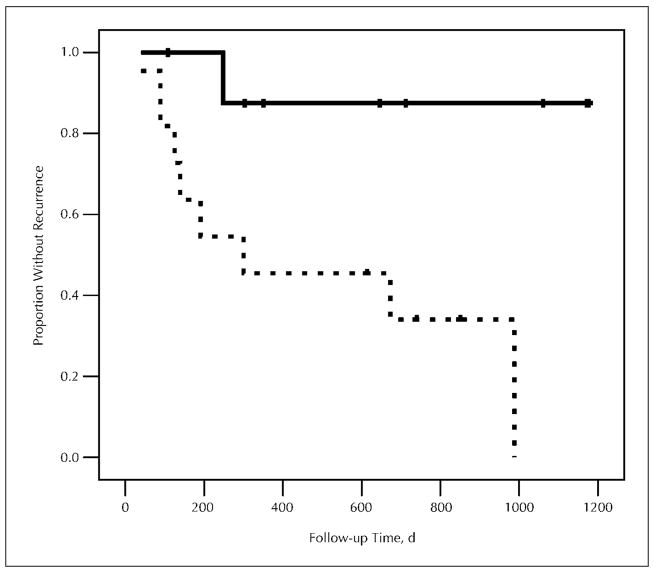
Patients found to have HCC in liver explants were followed for a median of 711 days (range, 108–2163 days) after OLT. Nine (45%) of 20 patients developed evidence of recurrent HCC, with a median time to recurrence of 191 days (range, 40–988 days). Eight of 11 patients with expression of VM in explanted tumor tissue developed recurrent HCC posttransplant, whereas only 1 of 9 patients without VM had recurrent HCC. The expression of VM in primary HCC lesions was associated with a shorter time to posttransplant recurrence (P = .01) (Figure 4). In multivariate analysis using Cox regression, potentially confounding factors were analyzed individually in conjunction with VM. The association between VM expression and more rapid tumor recurrence persisted when controlling for age, sex, cause of liver disease, tumor size, grade, and stage.
Figure 4.
Kaplan-Meier plot showing time to recurrence in patients with vasculogenic mimicry (dashed line) and without vasculogenic mimicry (solid line) expression by laminin stain. Patients with vasculogenic mimicry expression had a more rapid hepatocellular carcinoma recurrence after liver transplantation (P = .01).
VM Expression and EGFR, VEGF, and CD31 Immunoreactivity
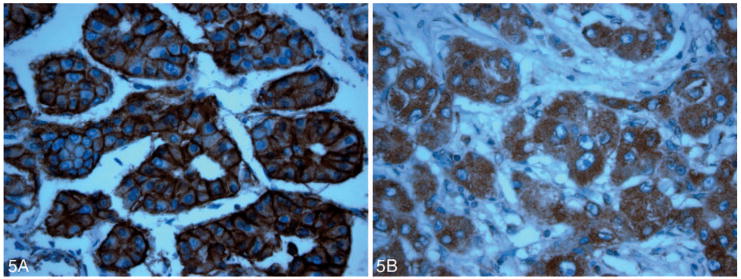
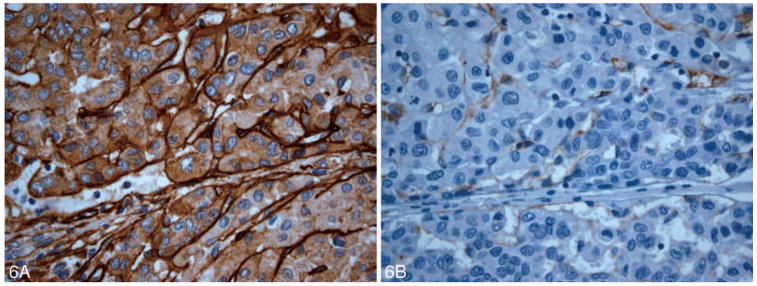
There were no significant associations among the following parameters: VM and EGFR staining intensity index (270 [100–300]; P = .36) or VM and VEGF staining intensity index (240 [100–300]; P = .89) (Figure 5, A and B). All hepatocytes in HCC lesions and control tissues were negative for CD31 (Figure 6, A and B).
Figure 5.
A, 3+ membranous positivity by epidermal growth factor receptor immunostain is identified in this hepatocellular carcinoma lesion (original magnification ×40). B, This hepatocellular carcinoma lesion shows 3+ cytoplasmic vascular endothelial growth factor positivity (original magnification ×40).
Figure 6.
A, Vasculogenic mimicry expression is identified by laminin immunostain in this hepatocellular carcinoma lesion that recurred rapidly (original magnification ×40). B, The same hepatocellular carcinoma lesion in A showing vasculogenic mimicry expression by laminin immunostain did not express the endothelial cell marker CD31 (original magnification ×40).
COMMENT
The current study provides the first evidence of patterned matrix VM in human HCC. We found VM in 55% of HCC specimens from 20 liver explants. Patterned matrix VM was associated with aggressive HCC, reflected by more rapid HCC recurrence after OLT. These retrospective findings suggest that staining for VM could have prognostic value in OLT candidates with HCC.
In the normal liver, laminin is present in the basement membranes of bile ducts, bile ductules, and vessels. In this pilot study we found that VM was not identified in normal hepatocytes, or in the hepatic parenchyma in chronic liver disease or cirrhosis. However, patterned matrix VM was easy to identify in HCC specimens by laminin immunostaining. Patterned matrix VM was distinct from angiogenic vessels on light microscopy because endothelial cell lined vessels do not form back-to-back loops in 2-dimensional histologic sections.1
We performed immunostaining for EGFR, VEGF, and CD31 to further evaluate whether changes of VM in HCC were associated with cell growth and cell survival or with endothelial markers. Epidermal growth factor receptor is a 170-kd transmembrane glycoprotein receptor with intrinsic tyrosine kinase activity that regulates cell growth, differentiation, and survival.27 Vascular endothelial growth factor stimulates proliferation of endothelial cells through specific tyrosine kinase receptors, flt-1 and flt/KDR, and is a central regulator of the angiogenic process.28,29 CD31 is a platelet endothelial cell adhesion protein molecule and is an indicator for angiogenesis in many tumors including hepatocellular carcinoma.30 In the current study, there were no significant associations between VM and expression of EGFR or endothelial cell markers VEGF and CD31. These results provide further evidence that VM expression in HCC is distinct from endothelial lined vessels.
The physical features of HCC that have been conventionally associated with HCC recurrence include the size and number of tumor nodules, microvascular/macrovascular invasion, and high serum α-fetoprotein levels.31 In a previous study, we found a significant association between a serum α-fetoprotein level greater than or equal to 100 ng/mL at time of diagnosis pretransplant and shorter time to recurrent HCC (P = .003).32 In the current study, there was no significant association between VM expression and serum α-fetoprotein level at the time of diagnosis or physical characteristics of the tumor. Moreover, the cause of liver disease (viral vs nonviral) was not associated with VM. Therefore, the formation of VM does not appear to be dependent on the cause of liver disease. It is likely that the formation of VM in biologically aggressive HCC is a host survival mechanism and has a dual effect of containing the tumor in a biological “cocoon,” thereby slowing its growth and metastasis, but could also potentially curtail the effectiveness of chemotherapy.
This pilot study was retrospective and the sample size was relatively small, limiting the power to detect differences between groups. The posttransplant recurrence rate was quite high. The recurrence rate appears to be related in part to the inclusion of 10 living donor recipients with HCC who were fast-tracked to transplantation. As a point of comparison, 42% of patients who underwent living donor liver transplantation for HCC in the multicenter adult-to-adult living donor liver transplantation (A2ALL) cohort study developed recurrence at 3 years.33 Nevertheless, our study population was followed in a consistent manner for tumor recurrence, which provides a clear clinical endpoint.
It is likely that VM in HCC was observed as early as 1982 by Sell and Ruoslahti34 in an immunoflourescence study that addressed the distribution of laminin in F344 rat livers. They described that laminin was predominantly observed in the basement membrane of blood vessels and bile ducts and inconsistently seen in sinusoids. Following treatment with carcinogens, laminin staining was associated with newly formed ductlike structures and oval cells, now known to be hepatic stem cells.34
In the current study, we identified vasculogenic mimicry in HCC and that it was associated with a more aggressive course of HCC after OLT. These data, subject to validation in larger patient samples, suggest that the histologic detection of VM could be added to the list of parameters used to predict the clinical course of HCC.
Acknowledgments
Supported by grant EY10457, National Institutes of Health, Bethesda, Md.
Footnotes
The authors have no relevant financial interest in the products or companies described in this article.
References
- 1.Folberg R, Maniotis AJ. Vasculogenic mimicry. Apmis. 2004;112:508–525. doi: 10.1111/j.1600-0463.2004.apm11207-0810.x. [DOI] [PubMed] [Google Scholar]
- 2.Clarijs R, Otte-Holler I, Ruiter DJ, de Waal RM. Presence of a fluid-conducting meshwork in xenografted cutaneous and primary human uveal melanoma. Invest Ophthalmol Vis Sci. 2002;43:912–918. [PubMed] [Google Scholar]
- 3.Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Molecular plasticity of human melanoma cells. Oncogene. 2003;22:3070–3075. doi: 10.1038/sj.onc.1206447. [DOI] [PubMed] [Google Scholar]
- 4.Maniotis AJ, Folberg R, Hess A, et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155:739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Massi D, Franchi A, Paglierani M, et al. Vasculogenic mimicry has no prognostic significance in pT3 and pT4 cutaneous melanoma. Hum Pathol. 2004;35:496–502. doi: 10.1016/j.humpath.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 6.Sun BC, Zhang SW, Zhao XL, Hao XS. Study on vasculogenic mimicry in malignant melanoma [in Chinese] Zhonghua Bing Li Xue Za Zhi. 2003;32:539–543. [PubMed] [Google Scholar]
- 7.Hendrix MJ, Seftor EA, Kirschmann DA, Seftor RE. Molecular biology of breast cancer metastasis: molecular expression of vascular markers by aggressive breast cancer cells. Breast Cancer Res. 2000;2:417–422. doi: 10.1186/bcr88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sood AK, Fletcher MS, Coffin JE, et al. Functional role of matrix metalloproteinases in ovarian tumor cell plasticity. Am J Obstet Gynecol. 2004;190:899–909. doi: 10.1016/j.ajog.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Sood AK, Seftor EA, Fletcher MS, et al. Molecular determinants of ovarian cancer plasticity. Am J Pathol. 2001;158:1279–1288. doi: 10.1016/S0002-9440(10)64079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu C, Huang H, Donate F, et al. Prostate-specific membrane antigen directed selective thrombotic infarction of tumors. Cancer Res. 2002;62:5470–5475. [PubMed] [Google Scholar]
- 11.Sharma N, Seftor RE, Seftor EA, et al. Prostatic tumor cell plasticity involves cooperative interactions of distinct phenotypic subpopulations: role in vasculogenic mimicry. Prostate. 2002;50:189–201. doi: 10.1002/pros.10048. [DOI] [PubMed] [Google Scholar]
- 12.Hao X, Sun B, Zhang S, Zhao X. Microarray study of vasculogenic mimicry in bi-directional differentiation malignant tumor [in Chinese] Zhonghua Yi Xue Za Zhi. 2002;82:1298–1302. [PubMed] [Google Scholar]
- 13.Sun B, Zhang S, Zhao X, Zhang W, Hao X. Vasculogenic mimicry is associated with poor survival in patients with mesothelial sarcomas and alveolar rhabdomyosarcomas. Int J Oncol. 2004;25:1609–1614. [PubMed] [Google Scholar]
- 14.Cai XS, Jia YW, Mei J, Tang RY. Tumor blood vessels formation in osteosarcoma: vasculogenesis mimicry. Chin Med J (Engl) 2004;117:94–98. [PubMed] [Google Scholar]
- 15.Lloyd RV, Vidal S, Horvath E, Kovacs K, Scheithauer B. Angiogenesis in normal and neoplastic pituitary tissues. Microsc Res Tech. 2003;60:244–250. doi: 10.1002/jemt.10263. [DOI] [PubMed] [Google Scholar]
- 16.Folberg R, Hendrix MJ, Maniotis AJ. Vasculogenic mimicry and tumor angiogenesis. Am J Pathol. 2000;156:361–381. doi: 10.1016/S0002-9440(10)64739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hess AR, Seftor EA, Gardner LM, et al. Molecular regulation of tumor cell vasculogenic mimicry by tyrosine phosphorylation: role of epithelial cell kinase (Eck/EphA2) Cancer Res. 2001;61:3250–3255. [PubMed] [Google Scholar]
- 18.Dupuy E, Hainaud P, Villemain A, et al. Tumoral angiogenesis and tissue factor expression during hepatocellular carcinoma progression in a transgenic mouse model. J Hepatol. 2003;38:793–802. doi: 10.1016/s0168-8278(03)00086-2. [DOI] [PubMed] [Google Scholar]
- 19.Zhao XL, Du J, Zhang SW, Liu YX, Wang X, Sun BC. A study on vasculogenic mimicry in hepatocellular carcinoma [in Chinese] Zhonghua Gan Zang Bing Za Zhi. 2006;14:41–4. [PubMed] [Google Scholar]
- 20.Sun B, Zhang S, Zhang D, et al. Vasculogenic mimicry is associated with high tumor grade, invasion and metastasis, and short survival in patients with hepatocellular carcinoma. Oncol Rep. 2006;16:693–698. [PubMed] [Google Scholar]
- 21.Gotoh M, Sakamoto M, Kanetaka K, Chuuma M, Hirohashi S. Overexpression of osteopontin in hepatocellular carcinoma. Pathol Int. 2002;52:19–24. doi: 10.1046/j.1440-1827.2002.01316.x. [DOI] [PubMed] [Google Scholar]
- 22.Anthony PP. Precursor lesions for liver cancer in humans. Cancer Res. 1976;36(7 pt 2):2579–2583. [PubMed] [Google Scholar]
- 23.Anthony PP, Vogel CL, Barker LF. Liver cell dysplasia: a premalignant condition. J Clin Pathol. 1973;26:217–223. [PMC free article] [PubMed] [Google Scholar]
- 24.Hirohashi S, Ishak K, Kojiro M, et al. Hepatocellular carcinoma. In: Hamilton SR, Aaltonen LA, editors. Pathology and Genetics of Tumours of the Digestive System. Lyon, France: IARC Press; 2000. pp. 157–172. World Health Organization Classification of Tumours. [Google Scholar]
- 25.Jiang Z, Wu CL, Woda BA, et al. Alpha-methylacyl-CoA racemase: a multi-institutional study of a new prostate cancer marker. Histopathology. 2004;45:218–225. doi: 10.1111/j.1365-2559.2004.01930.x. [DOI] [PubMed] [Google Scholar]
- 26.Luo J, Zha S, Gage WR, et al. Alpha-methylacyl-CoA racemase: a new molecular marker for prostate cancer. Cancer Res. 2002;62:2220–2226. [PubMed] [Google Scholar]
- 27.Matsuo M, Sakurai H, Saiki I. ZD1839, a selective epidermal growth factor receptor tyrosine kinase inhibitor, shows antimetastatic activity using a hepatocellular carcinoma model. Mol Cancer Ther. 2003;2:557–561. [PubMed] [Google Scholar]
- 28.Fox SB, Turley H, Cheale M, et al. Phosphorylated KDR is expressed in the neoplastic and stromal elements of human renal tumours and shuttles from cell membrane to nucleus. J Pathol. 2004;202:313–320. doi: 10.1002/path.1520. [DOI] [PubMed] [Google Scholar]
- 29.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 30.Ruck P, Xiao JC, Kaiserling E. Immunoreactivity of sinusoids in hepatocellular carcinoma: an immunohistochemical study using lectin UEA-1 and antibodies against endothelial markers, including CD34. Arch Pathol Lab Med. 1995;119:173–178. [PubMed] [Google Scholar]
- 31.Nakanishi K, Sakamoto M, Yamasaki S, Todo S, Hirohashi S. Akt phosphorylation is a risk factor for early disease recurrence and poor prognosis in hepatocellular carcinoma. Cancer. 2005;103:307–312. doi: 10.1002/cncr.20774. [DOI] [PubMed] [Google Scholar]
- 32.Guzman G, Alagiozian-Angelova V, Layden-Almer JE, et al. p53, Ki-67, and serum alpha feto-protein as predictors of hepatocellular carcinoma recurrence in liver transplant patients. Mod Pathol. 2005;18:1498–1503. doi: 10.1038/modpathol.3800458. [DOI] [PubMed] [Google Scholar]
- 33.Kulik L, Abecassis M. Living donor liver transplantation for hepatocellular carcinoma. Gastroenterology. 2004;127(5 suppl 1):S277–S282. doi: 10.1053/j.gastro.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 34.Sell S, Ruoslahti E. Expression of fibronectin and laminin in the rat liver after partial hepatectomy, during carcinogenesis, and in transplantable hepatocellular carcinomas. J Natl Cancer Inst. 1982;69:1105–1114. [PubMed] [Google Scholar]