Abstract
The growth factors neurotrophin 4 (NT4) and brain-derived neurotrophic factor (BDNF) are expressed in the developing skin, activate the trkB tyrosine kinase receptor, and influence the development and survival of specific types of sensory afferents. Whether each factor is capable of regulating the same or overlapping populations of cutaneous afferents during development is unknown. A previous study of mice overexpressing BDNF in the developing skin (BDNF-OE mice) revealed that these animals exhibited increased hair follicle innervation, Meissner corpuscle size, and Merkel cell number in glabrous skin although no change in the total number of sensory neurons was observed. To determine if NT4 affects cutaneous innervation in a manner similar to BDNF, transgenic mice overexpressing NT4 in skin, under the control of the keratin 14 gene promoter, were examined. Similar to BDNF-OE mice, NT4-OE mice had increased innervation to the skin but no increase in sensory neuron number in either the dorsal root ganglion or trigeminal ganglion. NT4 overexpression also enhanced hair follicle innervation and the size and density of innervation to Meissner corpuscles. Unlike BDNF overexpression, NT4 overexpression did not alter the number of Merkel cells in the glabrous skin, but it did enhance the number of myelinated axons in nerves projecting to skin. Thus, the same pattern of BDNF and NT4 overexpression within the skin produces phenotypes that are both similar and distinctive.
Indexing terms: sensory, neurotrophin, development
INTRODUCTION
Neuronal target tissues such as the skin produce several neurotrophic growth factors that regulate neuron survival, differentiation, and sensory end organ development. Two of these growth factors, brain-derived neurotrophic factor (BDNF) and neurotrophin-4 (NT4), bind and activate the tyrosine kinase receptor trkB and the p75 neurotrophin receptor (p75NTR). Although BDNF and NT4 signal through the same receptor, mutant mice lacking BDNF or NT4 exhibit some differences in phenotype. For example, the absence of BDNF during development results in a 21–44% reduction in trigeminal and 30–36% loss in dorsal root ganglion neurons (DRG), while no neurons are lost from the DRG or trigeminal ganglion in NT4 knockout mice (Jones et al., 1994; Conover et al., 1995; Liu et al., 1995; Liebl et al., 1997). However, NT4, not BDNF, is important for the development of a specific subpopulation of D-hair afferents (Stucky et al., 1998). The observed differences in BDNF and NT4 deficient mice could be due to dissimilar in vivo expression patterns or differences in the biological activities of BDNF and NT4 downstream of receptor binding. In NT4 knock-in mutants in which the BDNF coding sequence is replaced with the NT4 sequence, placodally derived sensory neurons are completely rescued (Fan et al., 2000), suggesting that NT4 and BDNF function in similar manners. However, NT4 knock-in mutants also display a number of abnormalities, including skin lesions. This suggests that BDNF and NT4 have unique functional properties with specific regard to sensory innervation of skin.
Transgenic mice overexpressing cDNAs encoding either NGF, NT3, or BDNF in the skin under control of the keratin 14 gene promoter have been particularly useful for examining the role of neurotrophins in the regulation of skin-innervation and sensory end-organ development (Albers et al., 1994; Albers et al., 1996; Davis et al., 1997; Rice et al., 1998; LeMaster et al., 1999; Krimm et al., 2004). In mice overexpressing NGF, the total number of trigeminal neurons was doubled, the skin was hyperinnervated by unmyelinated fibers, and trkA-positive nociceptors were enhanced in number and displayed increased sensitivity (Albers et al., 1994; Davis et al., 1997; Stucky et al., 1999). Mice overexpressing NT3 in the skin had 65% more trigeminal neurons, a doubling of trkC-positive neurons, and larger touch dome mechanoreceptors which contained more Merkel cells upon which NT3-dependent slowly adapting type I neurons terminated (Albers et al., 1996; Krimm et al., 2000; Krimm et al., 2004). Therefore, expression of NGF or NT3 in the skin regulates sensory neuron survival and each neurotrophin influences a distinct subpopulation of trigeminal and DRG afferents.
The effects of BDNF on cutaneous afferents have also been investigated using mice that overexpress BDNF in the skin. BDNF-OE mice exhibited hyperinnervation of hair follicles, enlarged Meissner corpuscles, and increased numbers of Merkel cells in glabrous skin, but they did not exhibit a change in the total number of trigeminal or DRG neurons (LeMaster et al., 1999). Thus, unlike NGF and NT3, overexpression of BDNF in the skin promoted innervation and enhanced cutaneous end organs without influencing sensory neuron number. Whether NT4 acts similarly to BDNF was the focus of the present investigation. To compare the function of NT4 in skin with that of BDNF, transgenic mice overexpressing NT4 under the control of the keratin 14 promoter were examined. The results indicate that NT4 affected cutaneous afferents similarly to BDNF though some unique changes in innervation were identified. Thus, the same pattern of BDNF and NT4 overexpression in the skin can produce distinct phenotypes suggesting that BDNF and NT4 function in both similar and independent manners.
METHODS
Animals
Adult (3–4 months of age) transgenic mice overexpressing NT4 in the basal cells of the epidermis and littermate wild type mice were examined. NT4 cDNA expression was driven by 2.3 Kb of the human keratin-14 promoter (Albers et al., 1994; (Krimm et al., 2001a). The K14-NT4 transgene was microinjected into fertilized embryos derived from C57Bl6J/C3H F1 hybrid females (Harlan). Of the two founder lines generated (86 and 516), 516 was chosen for further analysis based on its higher level of transgene expression. Genotypes were determined using transgene specific PCR primers and/or slot blot hybridization using a 32P-labeled probe to the K14-NT4 transgene. The colony was maintained by breeding transgenic mice to wild type mice of the same background such that comparisons were always made between NT4-OE mice and wild type littermates. Animals were cared for and used in accordance with guidelines of the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals and the NIH Guide for the Care and Use of Laboratory Animals.
Two-site enzyme-linked immunosorbant assay of NT4 protein
ELISAs were performed using the NT4 Emax Immunoassay kit (Cat#G4890, Promega, Madison, WI) according to the manufacturer’s instructions. The anti-NT4 antibody used was a polyclonal rabbit antibody made against the entire recombinant human NT4 sequence. The antibody has not been tested on a Western blot; however, it was determined to have minimal cross-reactivity to related neurotrophic factors on dot blots and on ELISA standard curves (see http://www.promega.com/nnotes/nn102/102_10.pdf for details). Mice were euthanized by anesthetic overdose, and a shaved piece of flank skin approximately 1cm square was removed, weighed, and frozen on dry ice. Skin was homogenized in sample buffer (0.1 M phosphate-buffer, 0.4 M NaCl, 0.1% Triton X-100, 2 mM EDTA, 0.1 mM benzethonium chloride, 2 mM benzamidine, 0.1 mM PMSF, 20 µl/ml aprotinin, 0.5% BSA, pH 7.4) using a polytron homogenizer. Samples were centrifuged at 13,000 rpm for 15 min at 4°C and aliquots of the collected supernatant assayed.
Histology and immunohistochemistry
NT4-OE and wild type littermates were deeply anesthetized with 2.5% avertin diluted in 0.9% saline and perfused with 4% paraformaldehyde in 0.1 M phosphate buffer. For hematoxylin and eosin (H&E) staining, the skin was post-fixed in 4% paraformaldehyde and 70% ethanol, embedded in paraffin, sectioned, and stained. For immunohistochemical labeling of neural fibers, mice were perfused with 4% paraformaldehyde and tissues were embedded in 10% gelatin that was post-fixed overnight in 4% paraformaldehyde. Gelatin-embedded tissue was infiltrated with 30% sucrose in phosphate buffered saline (PBS), frozen, and sectioned at 40 µm on a sliding microtome. Tissue sections were washed in PBS, blocked for 1hr at room temperature in 5% normal goat serum (NGS), 2% bovine serum albumin (BSA), and 0.25% Triton X-100. Sections were then incubated overnight in either anti-neurofilament 150 (rabbit polyclonal, used 1:1000; antigen: highly purified bovine neurofilament polypeptide, Cat# AB1981, Chemicon, Temecula, CA; antibody shows a prominent 150kDa band, and a few bands at or below 48kDa on a Western blot) or anti-PGP9.5 (rabbit polyclonal,1:5000; antigen: human PGP9.5 protein purified from pathogen free human brain, RA95101, Ultraclone, Isle of Wight, UK; antibody shows one band at 26-28kDa on Western blot), diluted in 5% NGS and 0.25% Triton X-100 made in PBS. Sections were then washed, incubated with 1:500 dilution of goat anti-rabbit biotinylated antibody (Vector Laboratories, Burlingame, CA) for 1 hr, re-washed, and treated with anti-endogenous peroxidase solution containing 2.5% H202 and 5% methanol. For antibody detection, tissues were incubated in an avidin-biotin-complex mix (ABC, Vectastain Elite; Vector Laboratories) for 1hr, washed, incubated in nickel enhanced-0.04% diaminobenzene solution, mounted onto slides, and counter stained with methyl-green. Labeling with both anti-neurofilament 150 and anti-PGP9.5 resulted in a similar distribution and morphology of labeled neuronal fibers as reported previously (Albers et al., 1994; Albers et al., 1996; LeMaster et al., 1999). To label Merkel cells of glabrous skin, skin containing all 5 footpads was removed, embedded in OCT compound on dry ice, and then sectioned on a cryostat at 20 µm thickness. Sections were then mounted on slides, fixed in −20°C acetone for 10 min., air dried, washed in PBS, and incubated with anti-cytokeratin #20 (mouse monoclonal, 1:20; immunogen: electrophoretically purified human keratin 20 from intestinal mucosa, Cat# M7019, DakoCytomation, Carpinteria, CA; there is no Western blot data available for this anti-body it was specifically designed as an immunohistochemical marker for Merkel cells and Merkel cell tumors) and anti-neurofilament 150 overnight at room temperature. Sections were washed and incubated in goat anti-rabbit Cy2 and goat anti-mouse Cy3 (Jackson Laboratory) for 1.5–2 hours, washed, and coverslipped with DPX mounting media (DBH Laboratory Supplies, Poole, England). Merkel cells were counted in serial sections of the entire footpad region. Anti-cytokeratin 20 is a well established marker for Merkel cells (Moll et al., 1995; Botchkarev et al., 1999). We observed a similar cellular distribution as previous studies that used anti-cytokeratin 20 (LeMaster et al., 1999; Krimm et al., 2004) as a Merkel cell marker. The distribution was also similar to earlier studies that used other Merkel cell markers (Kinkelin et al., 1999; Harada et al., 2000; Szeder et al., 2003).
Neuron cell counts
The number of neurons in adult L4/L5 DRG and trigeminal ganglia were determined using methods described by Coggeshall et al. (1990) Ganglia were serially cut into 5 µm-thick sections, Nissl stained, and every 10th section examined at 400X magnification to identify neurons with a nucleolus. We estimated total neuron number by adding all the nucleoli counted across sections and multiplying by 10. To compensate for neurons with two or more nucleoli or split nucleoli, approximately 100 nuclei/ganglion were serially reconstructed and the number of nucleoli per neuron nucleus was determined. Neurons were reconstructed from serial sections near the middle of the ganglion, all cells with nuclei in selected sections were reconstructed. This correction factor was multiplied by the total neurons counted to obtain the total number of neurons per ganglion.
Electron microscopy
Mice were deeply anesthetized and perfused with 0.1M phosphate buffered saline followed by 4% paraformaldehyde made in phosphate buffer. Saphenous nerve segments were removed from mid-thigh level, post-fixed for 2 h in 2% gluteraldehyde and 4% paraformadehyde, washed in phosphate buffer, dehydrated in alcohols, embedded in Spurr’s resin, and cut to a 0.7–0.8 nm thickness on an ultramicrotome. Ultrathin sections were stained and photographed to create montages of the entire nerve cross section from which the number of myelinated and unmyelinated nerve fibers was determined. Diameters of myelinated fibers were measured using NIH image software. Areas of 200 myelinated fibers randomly selected from each nerve sample (n=3/genotype) were determined.
In situ mRNA hybridization
In situ hybridization to detect trk and p75 receptor mRNA using 35S-labeled probes was carried out as described previously (Albers et al., 1994). Sense and antisense RNA riboprobes were generated to cDNA sequences encoding the rat trkA, trkB, and trkC receptors (Kitzman et al., 1998; LeMaster et al., 1999). DRG and trigeminal ganglia were frozen on dry ice, cut into 15 µm sections, and thaw mounted onto Superfrost slides. Sections were brought to room temperature, immersion-fixed for 15 min in 4% paraformaldehyde in PBS, and then washed in PBS, PBS with 0.2% glycine, and 0.25% acetic anhydride in 0.1 M TEA, pH 8.0. Sections were dehydrated, air-dried, and incubated with probe hybridization solution (Amresco, Solon, OH) containing 1 × 106 cpm/50 µl. A glass coverslip was placed over the probe and secured to the slide by applying mounting media on the edges of the cover glass. Slides were incubated overnight at 60°C, dipped in NTB2 photographic emulsion, exposed 1–2 weeks, developed, and counterstained with hematoxylin and eosin. Sense transcript controls processed in parallel showed no specific hybridization. The number of labeled and unlabeled neuron profiles was quantified in 3 different sections containing the combined L4/L5 DRG or in 2 sections containing trigeminal ganglion. The area of each profile was measured and the diameters were calculated from area measurements. The number of neurons in each section was determined by multiplying the number of profiles by an Abercrombie correction factor, which was the section thickness divided by the section thickness plus the mean neuron diameter. The percent of labeled neurons per section was determined from these corrected values and averaged across sections. The mean percent labeled neuron profiles and the average diameters for each trk receptor and p75 are shown in table 4 (n=4).
Table 4. Percent and mean diameter of trk receptor labeled neurons in sensory ganglia.
In situ hybridization was performed on wild type and NT4-OE dorsal root and trigeminal ganglia using 35S-labeled riboprobes to either trkA, trkB, or trkC mRNA. Labeled and unlabeled neuron profiles were counted in 3 randomly selected sections per ganglia, and the diameters for each profile was also measured. An Abercrombie conversion factor was used to convert profile counts to neurons. Labeled neurons are expressed as the percent of total neurons. Statistical significance was determined using a t-test.
| DRG | Trk A | Trk B | Trk C |
|---|---|---|---|
| % of total | |||
| Wild type (n=4) | -- | 23.7% | 41.6% |
| NT4-OE (n=4) | -- | 28.4%* | 30.2%* |
| Mean diameter | |||
| Wild type (n=4) | -- | 31.4µm | 29.1µm |
| NT4-OE (n=4) | -- | 35.1µm* | 30.0µm |
| Trigeminal % of total | |||
| Wild type (n=4) | 32.9% | 38.7% | -- |
| NT4-OE (n=4) | 28.6% | 76.6%* | -- |
| Mean diameter | |||
| Wild type (n=4) | 24.9µm | 26.2µm | -- |
| NT4-OE (n=4) | 26.9µm | 30.2µm | -- |
Asterisk indicates significance.
RESULTS
K14-NT4 transgenic mice express increased levels of NT4 peptide in the skin
NT4 expression was driven by the human keratin K14 promoter to direct high levels of transgene expression to the skin and tongue epithelium (Vassar and Fuchs, 1991; Albers et al., 1994; Wang et al., 1997). Promoter activity in whisker pad skin begins at approximately E11.5 of mouse development (Figueiredo et al., 2001) which precedes peak periods of developmental cell death in the DRG and trigeminal sensory ganglia. The transgene is constitutively expressed in basal keratinocytes into adulthood. To verify translation of the transgene mRNA, NT4 peptide was measured in dorsal skin of two transgenic lines using a NT4-specific ELISA. As shown in Table 1, NT4 peptide levels increased 1.8 -fold in line 87 (p< 0.05) and 2.3-fold in line 516 (p< 0.05). Because line 516 exhibited the greatest increase in NT4 protein, subsequent analyses were limited to this line.
Table 1. NT4 protein level in dorsal skin as measured by ELISA.
NT4 protein levels were measured in dorsal skin using an ELISA (nanograms per grams wet weight). Levels of NT4 were significantly increased in the dorsal skin of both line 516 (n=5) and line 87 (n=3). Statistical significance was determined using a t-test; values are mean ± SEM.
| NT4 Line 516 | NT4 Line 87 | |||
|---|---|---|---|---|
| NT4 transgenic | Wild type | NT4 transgenic | Wild type | |
| Protein (ng/g) | 10.95 ± 1.28* | 4.70 ± .571 | 26.59 ± 2.82* | 14.61 ± 2.77 |
| Increase | 2.3 | 1 | 1.8 | 1 |
Asterisk indicates significance (p < 0.05).
NT4 overexpression increases innervation to the skin and enhances sensory end organ development
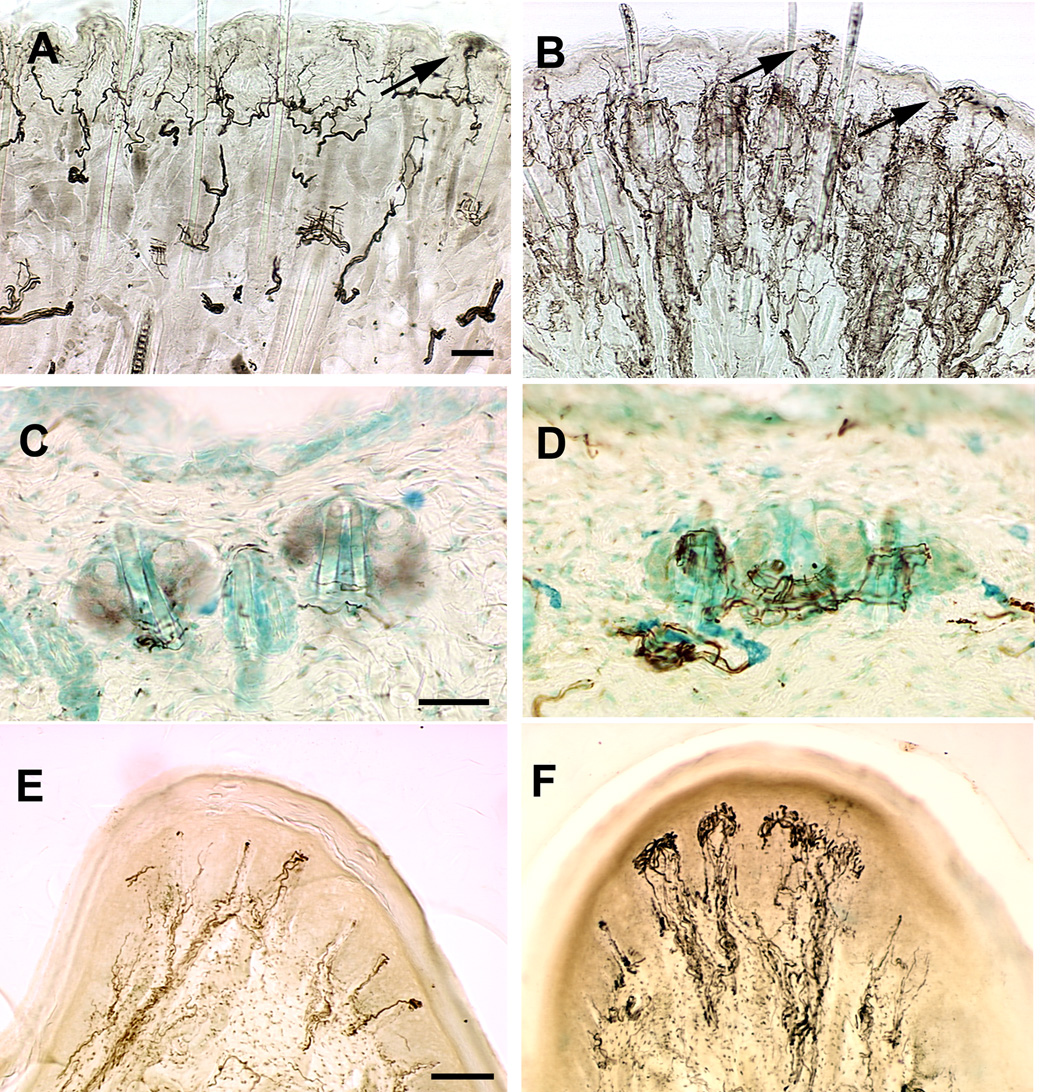
To determine whether NT4 overexpression affected skin innervation, we analyzed adult whisker pad, flank, and footpad skin. Immunohistochemical labeling with anti-PGP9.5 showed increased innervation in whisker pad skin of NT4-OE mice compared with their wild type counterparts (Fig. 1 A, B). Innervation was particularly robust in fibers surrounding hair follicles, an enhancement also evident in flank skin (Fig. 1 C, D). These endings may be of the D-hair variety, whose survival and function have been shown to be regulated by NT4 (Stucky et al., 1998). This enhancement is similar to that observed in mice overexpressing BDNF in skin (BDNF-OE) (LeMaster et al., 1999). Neural innervation to the whisker pad appeared retracted from the skin’s surface in BDNF-OE mice during postnatal development. In contrast, retraction of nerve fibers from the NT-4-OE epithelium was not apparent (Fig. 1 A, B).
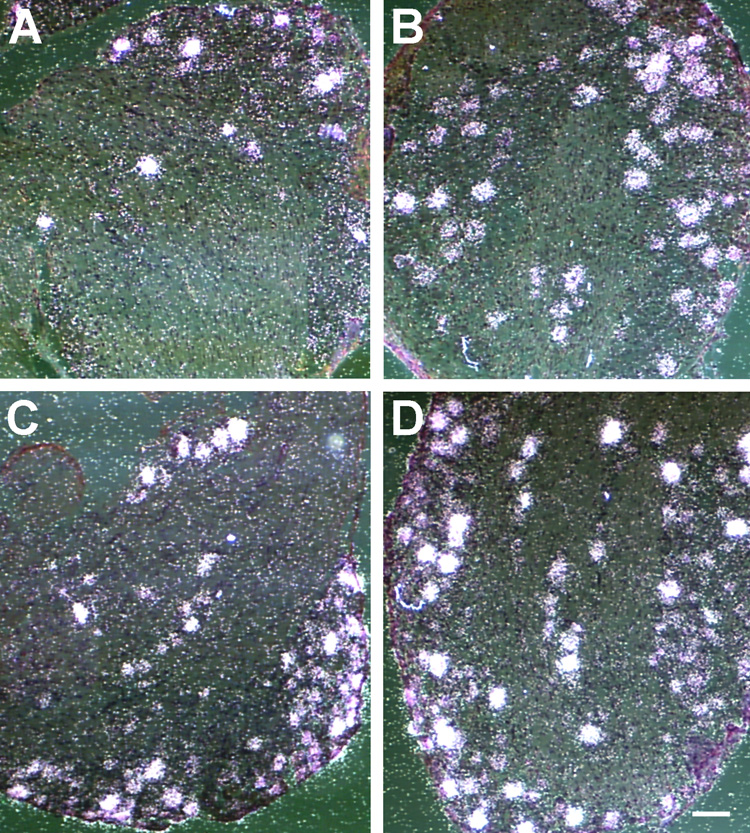
Figure 1.
Neural innervation of skin is enhanced in NT4-OE transgenic mice. Whisker pad (A, B), flank (C, D) and footpad (E, F) skin in wild type (A, C, E) and NT4-OE (B, D, F) mice was labeled using an antibody to PGP9.5. Hyperinnervation was evident around hair follicles in NT4-OE mice (B) compared with wild type mice (A). Arrows in A and B point to epidermal endings. Hair follicle innervation was also increased in flank skin of NT4-OE mice (D) compared with wild type mice (C). Anti-neurofilament labeling reveals an increased innervation of dermal papillae in the footpad of NT4-OE (F) mice compared with wild type mice (E). Scale bar in A = 50µm and applies to A and B. Scale bar in B = 50µm and applies to C and D. Scale bar in E = 100µm and applies to E and F. The brightness and contrast of all images were optimized to clarify the extent of innervation.
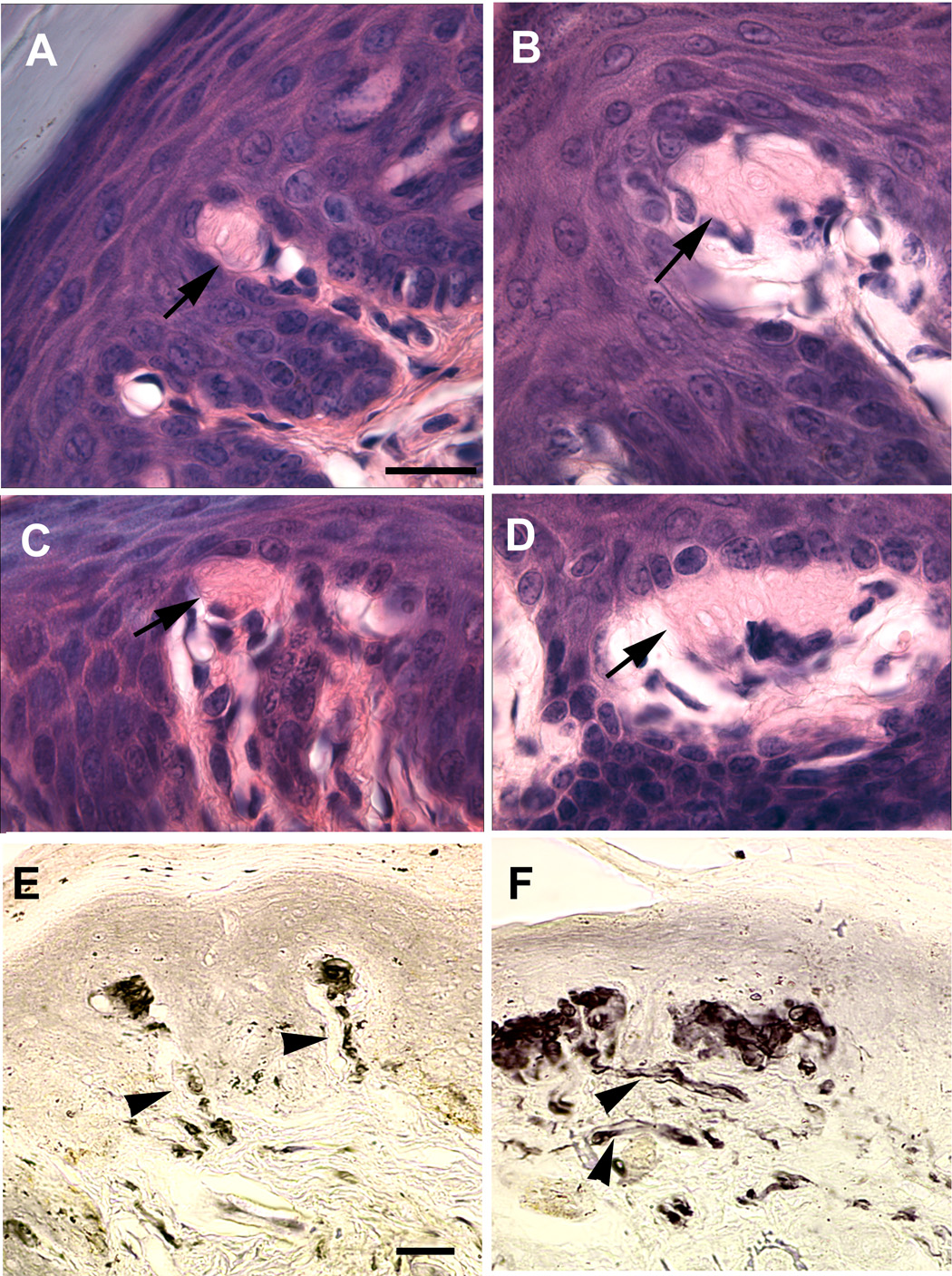
Innervation was also examined in non-hairy, glabrous skin of the front footpad (Fig. 1 E, F). Immunohistochemical labeling with anti-neurofilament antibody revealed a significant increase in the extent of myelinated innervation to dermal papillae of the footpad. An encapsulated sensory receptor end organ located in these papillae is the Meissner corpuscle. These endings have a distinctive lamellar structure and can be identified in hematoxylin and eosin (H&E) stained tissue sections of the foot. Using both H&E staining and anti-PGP9.5 immunohistochemical labeling, Meissner corpuscles in the footpads were found to be increased in size in NT4-OE mice (Fig. 2B, D, F) when compared those present in wild type mice (Fig. 2A, C, E). Some Meissner corpuscles in NT4-OE mice were exceptionally large and contained lamellar cells that appeared disorganized (Fig. 2C, D, E, F). These enhancements were also exhibited by BDNF-OE mice (LeMaster et al., 1999), suggesting that a common signaling mechanism underlies NT4 and BDNF modification of Meissner ending size.
Figure 2.
Meissner corpuscles in the footpad are enlarged in NT4-OE mice. Footpad from wild type (A, C) and NT4-OE mice (B, D) were stained with hematoxylin and eosin (H&E). The laminar encapsulated endings (arrows) were larger in NT4-OE mice in Meissner corpuscles found within the pad region (B compared with A) and between individual pads (compare D with C). In addition, H&E staining (D compared with C) and anti-PGP9.5 labeling (F compared with E) revealed that the organization of corpuscles located between pads was disrupted. Nerve bundles (arrowheads) to Meissner corpuscles of NT4-OE mice (F) also appeared larger compared to those of wild type mice (E). Scale bar in A = 20µm and applies to A–D; scale bar in E = 20µm and applies to E and F. The brightness and contrast of the image were optimized for visualization of Meissner corpuscles and the nerves associated with them.
Previous analysis of glabrous skin in BDNF-OE mice revealed an increase in the number of Merkel cells relative to the number in wild type mice (LeMaster et al., 1999). To determine if NT4 overexpression had a similar effect on glabrous Merkel cells, their number and innervation was assessed in NT4-OE and wild type footpad skin. Glabrous Merkel cell neurite complexes in NT4-OE mice were organized into both small (Fig. 3 A, B) and large (Figure 3 C, D) clusters in the footpads of both wild type (Fig. 3A, C) and NT4-OE (Fig. 3B, D) animals. There was no statistically significant difference in the number of Merkel cells between NT4-OE mice and wild type mice (882±16 vs. 897±18; p ≤ 0.57). Therefore, unlike BDNF, NT4 overexpression does not enhance the number of glabrous Merkel cells.
Figure 3.
Footpad Merkel complexes are not altered in NT4-OE mice. Footpads from wild type (A, C) and NT4-OE (B, D) mice were labeled with anti-cytokeratin 20 to detect Merkel cells (red) and with anti-neurofilament 150 (green) to detect nerve fibers. Merkel cells were organized in clusters subjacent to the epidermis and were innervated in all mice examined. No difference in either the number of Merkel cells or the amount of innervation to Merkel cell complexes was evident. The brightness and contrast was optimized across all images for visualization of both labels. Scale bar in D = 20µm and applies to all.
NT4-overexpression increases the number of myelinated afferents but not the number of neurons
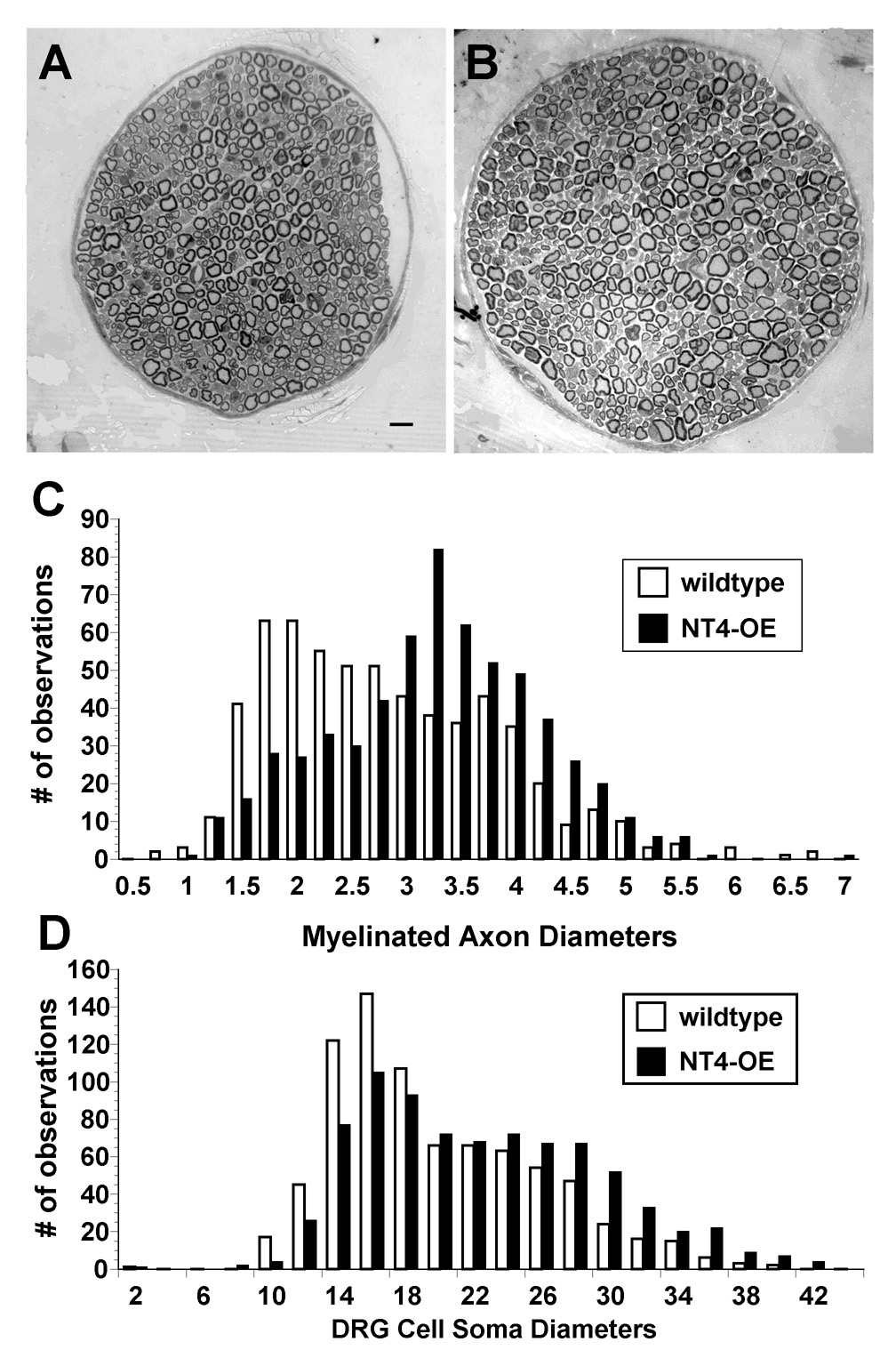
To determine whether the increased innervation observed in NT4-OE skin correlated with an increase in afferent projections to the skin, the number of fibers in the saphenous nerve, which is purely cutaneous, was counted (Fig. 4). In NT4-OE mice, the number of myelinated fibers increased by 36% while the number of unmyelinated fibers was unchanged (Table 2). Frequency histogram plots of the diameter of 150 myelinated axons in transgenic and wild type nerves revealed that transgenic afferents were also enlarged (Fig. 4C). Interestingly, although axon number was increased, the number of trigeminal and L4/L5 DRG neurons in NT4-OE mice were unchanged (Table 3). In comparison, as shown in Table 3, the number of neurons in the geniculate ganglion, a neuronal population known to be highly dependent on NT4 during development (Liu et al., 1995), was increased by 262% (Krimm et al., 2001a). Thus, NT4 overexpression increases survival of specific types of sensory neurons.
Figure 4.
Overexpression of NT4 enhances the size of the saphenous sensory nerve. NT4-OE mice (B) displayed larger saphenous nerves than wild type mice (A). (C) Diameters of 150 randomly chosen myelinated axons per animal (n=3 for NT4-OE and n=3 for wild type) were plotted against the corresponding number of times the diameter was observed in NT4-OE mice (black bars) and wild type mice (white bars). The number of myelinated axons (Table 2) and axon diameter were increased in NT4-OE mice (C). (D) Diameters of 200 randomly chosen DRG neurons per animal (n=3 for NT4-OE and n=3 for wild type) were plotted as described above for NT4-OE mice (black bars) and wild type mice (white bars).
Table 2. Number of axons in wild type and NT4-OE saphenous cutaneous nerves.
Values are for NT4 transgenic line 516. Sample size was n = 3 for each group. Statistical significance was determined using a t-test and are expressed as SEM.
| Myelinated nerve fibers | Unmyelinated nerve fibers | |||
|---|---|---|---|---|
| NT4 transgenic | Wild type | NT4 transgenic | Wild type | |
| Nerve count | 905 ± 20.2* | 583 ± 14.0 | 2906 ± 209.3 | 2666 ± 104.3 |
| Percent increase | 55 | NA | 0.09 | NA |
| Percent of total | 24 | 18 | 76 | 82 |
| nerve count | ||||
Asterisk indicates significance (p< 0.05).
Table 3. Neuronal counts of adult NT4-OE transgenic and wild type mice.
Statistical significance was determined using a t-test; values are expressed as SEM.
| Mouse genotype | Dorsal root ganglia (L4/L5) |
Trigeminal ganglia | Geniculate ganglia |
|---|---|---|---|
| Wild type | 12859 ± 1793 (n=4) | 40286 ± 2912 (n=3) | 799 ± 96 (n=3) |
| NT4 transgenic | 12710 ± 1019 (n=4) | 38296 ± 7020 (n=3) | 2097 ± 118* (n=3) |
Asterisk indicates significance (p<0.05).
How increased levels of NT4 in the skin enhanced the number of myelinated axons in the saphenous nerve without increasing the number of lumbar DRG neurons is unclear. One possible explanation is that increased levels of NT4 altered differentiation of developing DRG neurons and caused proprioceptive neurons projecting to muscle to switch fate to cutaneous afferents that project to skin. To test this hypothesis, we quantified the number of myelinated axons in two nerves that provide sensory innervation to muscle, the common peroneal and lateral gastocnemius nerves. Myelinated fibers in the common peroneal nerve of NT4-OE mice were slightly increased relative to wild type mice (957±58 vs. 735±32; p<0.04). No change in axon number was detected in the lateral gastrocnemius nerve of NT4-OE mice compared to wild type (170±8 vs. 211±39; p<0.4). Therefore, these data do not support the notion that NT4 overexpression causes muscle proprioceptors to differentiate into skin-innervating mechanoreceptors.
NT4-OE leads to trkB receptor up-regulation in the trigeminal and dorsal root ganglion
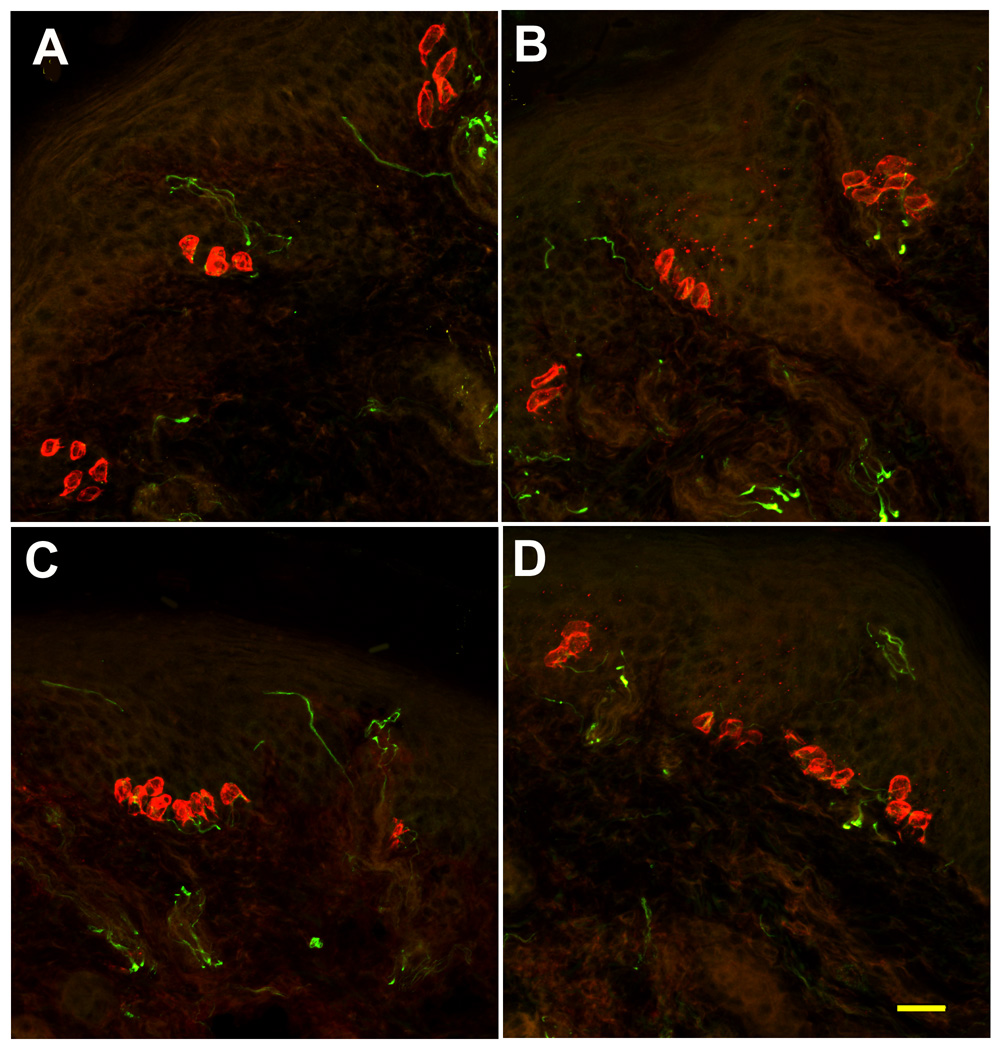
Neurotrophin signaling is mediated by receptor tyrosine kinases in a preferential manner such that trkA binds NGF, trkC binds NT3, and trkB binds BDNF and NT4. Previous analysis of BDNF-OE mice revealed a 60% increase in neurons expressing the trkB receptor (LeMaster et al., 1999). Because neuron number was unchanged in these mice, the increase in trkB neurons was hypothesized to reflect an up-regulation of trkB in neurons that are normally trkB-negative or express non-detectable levels of trkB. To determine whether NT4 overexpression may similarly influence trkB expression, in situ hybridization was employed to quantify the proportion of DRG neuron profiles expressing full-length trkB receptor mRNA. NT4-OE mice exhibited a 20% increase in trkB-positive neurons in the DRG (p<0.04, Table 4) and a 49% increase in the trigeminal ganglion (p<0.01; Table 4). Because the number of neurons did not increase in NT4-OE mice (Table 3), this increase in trkB is likely due to trkB up-regulation, similar to BDNF-OE mice. TrkB expressing neurons were also larger in NT4-OE mice than in wild type mice (p<0.006; Table 4). NT4-OE did not increase the size of TrkC expressing neurons although the mean diameter of trkB and trkC expressing neurons was larger than the mean diameter of unlabeled neurons in the same ganglion (p<.0002; p<.001). Especially interesting was the finding that overexpression of NT4 caused a decrease in the proportion of trkC expressing neurons, suggesting trkC expressing neurons were converted to a trkB expressing fate in NT4-OE mice. Overexpression of NT4 was not associated with any change in trkA expressing neurons in the trigeminal ganglion.
DISCUSSION
Previous studies of transgenic mice overexpressing BDNF in the skin demonstrated that BDNF is capable of enhancing the density of peripheral innervation and specific sensory receptors without increasing the number of neurons in the DRG or trigeminal ganglion (LeMaster et al., 1999). Because BDNF and NT4 both bind the trkB and p75 receptors, we investigated whether skin-derived NT4 functions similarly to BDNF by comparing the effects of NT4 overexpression in skin with previously reported effects of BDNF overexpression (Table 5). Our results show that both similarities and differences exist in the effects NT4 and BDNF overexpression have on sensory structures.
Table 5. Comparison of NT4-OE phenotype with BDNF-OE phenotype (reported in LeMaster et al., 1999).
Overexpression of NT4 in skin produces a phenotype with similarities and differences to the phenotype of mice that overexpress BDNF in skin.
| Phenotype | BDNF-OE | NT4-OE |
|---|---|---|
| Peripheral innervation | ||
| Hair follicle innervation | Increased | Increased |
| Meissner corpuscles | Increased | Increased |
| Merkel cell endings (footpad)* | Increased | No change |
| Saphenous axon number | ||
| Myelinated* | No change | Increased (55%) |
| Unmyelinated* | Decreased (24%) | No change |
| Neuron number | ||
| DRG | No change | No change |
| Trigeminal | No change | No change |
| Geniculate | Increased (223%) | Increased (262%) |
| Trk receptor expression–DRG | ||
| TrkB* | Increased (60%) | Increased (20%) |
| TrkC* | No change | Decreased (27%) |
| Trk receptor expression –trigeminal | ||
| TrkA* | Increased (70%) | No change |
| TrkB* | Increased (292%) | Increased (49%) |
Asterisk indicates difference in NT4-OE and BDNF-OE phenotypes.
Although peripheral innervation to the skin was increased in NT4-OE and BDNF-OE mice, we detected no difference in the number of Merkel cells in glabrous skin of NT4-OE mice. Thus, only BDNF overexpression appears to influence glabrous Merkel cell number (Botchkarev et al., 1999; LeMaster et al., 1999). This enhancement in glabrous Merkel cell number by BDNF is specific to these cells since Merkel cells of hairy skin respond to NT3 rather than BDNF (Airaksinen et al., 1996; Albers et al., 1996; LeMaster et al., 1999; Krimm et al., 2000; Krimm et al., 2004). The distinct neurotrophic requirements for different Merkel cell populations may be related to differences in the nerve-dependency of these populations (Mills et al., 1989). Merkel cells of hairy skin are nerve-dependent, and NT3 may act primarily by influencing the slowly adapting type 1 neurons that innervate them (Krimm et al., 2004). In contrast, glabrous Merkel cells are nerve-independent, and BDNF produced in the skin may exert a paracrine influence. It is unclear why NT4 overexpression cannot substitute for BDNF in this putatively non-neuronal mechanism of support for glabrous Merkel cells.
Enlargement of Meissner endings was observed in footpad skin of NT4-OE and BDNF-OE mice, suggesting that BDNF and NT4 function interchangeably to regulate the size and innervation pattern to Meissner endings. This finding is consistent with previous reports that Meissner corpuscles are dependent on trkB for their development (Ichikawa et al., 2000; Gonzalez-Martinez et al., 2004). Interestingly, recent studies of NT4 and BDNF gene knockout mice showed that NT4 is not required for Meissner corpuscle development, though BDNF is necessary (Gonzalez-Martinez et al., 2005). Thus, although NT4 can influence Meissner corpuscles via trkB, it is not essential for their development. NT4-OE mice also exhibit a substantial increase in myelinated innervation within dermal papilla of the footpad. While much of this increased innervation could be related to the enhanced Meissner complex, it may also reflect another, as yet undefined neural population. A subpopulation of sensory fibers in hairy skin that were influenced in a similar manner by both NT4 and BDNF were hair follicle afferents.Overexpression of both neurotrophins resulted in increased innervation to hair follicles in both whisker pad and back skin. While hair follicle innervation is not lost in the whisker pads of NT4 knockout mice (Fundin et al., 1997; Rice et al., 1998), much of this increased innervation in NT4-OE mouse back skin may reflect increases in down hair receptors, which are lost in mice lacking NT4 (Stucky et al., 1998).
Overexpression of either BDNF or NT4 led to increased innervation to the skin without increasing the number of neurons in DRG or trigeminal ganglia. In contrast, BDNF and NT4 caused a marked increase in survival of neurons in placode-derived ganglia (geniculate and nodose;(LeMaster et al., 1999; Krimm et al., 2001b). In this respect, skin-derived BDNF and NT4 function in a similar manner, consistent with studies demonstrating that NT4 knocked into the BDNF locus rescues sensory neurons in BDNF gene null mice (Fan et al., 2000). Despite these similarities in the regulation of sensory neuron number, only NT4 overexpression increased the number of myelinated fibers in the saphenous nerve. This difference suggests that these two trkB ligands increase innervation to skin via different mechanisms. That is, BDNF-mediated enhanced innervation to the skin may act locally; whereas, NT4 enhancement may act by regulating sensory axon number at the level of the nerve.
Interestingly, the number of myelinated nerve fibers in the cutaneous saphenous nerve increased in NT4-OE mice although the number of DRG neurons was unchanged. Similarly, mice lacking NT4 lose myelinated axons in the saphenous nerve without losing DRG neurons (Stucky et al., 1998; Liebl et al., 2000). One possible explanation for this finding is that neuron precursors destined to become mechanoreceptors of the skin, instead, differentiated into proprioceptors innervating muscle in these mice (Liebl et al., 2000). In NT4-OE mice, a reverse effect could have occurred such that DRG neurons differentiated into mechanoreceptors innervating the skin at the expense of muscle proprioceptors. Consistent with this possibility NT4-OE mice had fewer TrkC expressing neurons and more TrkB expressing neurons than wild type mice. This finding suggested that NT4 overexpression converted TrkC expressing proprioceptors into TrkB expressing mechanoreceptors. To test this hypothesis, we examined whether increases in NT4 converted NT3-dependent proprioceptive neurons innervating muscle to mechanoreceptors innervating skin (Liebl et al., 2000). If increased skin innervation was due to altered differentiation of NT3-dependent proprioceptors, fewer myelinated sensory fibers would innervate muscle. In fact, in the two sensory nerves examined that innervate muscle, the common peroneal and lateral gastocnemius nerves, the number of myelinated nerve fibers was either increased or unchanged. Therefore, altered neuronal differentiation does not account for the increase in skin innervation in NT4-OE mice.
A more likely explanation for the incongruity between cell number and axon number is that NT4-OE increases the branching of myelinated nerve fibers. Increased nerve fiber branching in the DRG of NGF-OE mice has been visualized by intercellular injection of a neuronal tracer into individual DRG neurons (H. Richard Koerber, personal communication). Similarly, enhanced NT4 levels may induce branching in a DRG subpopulation thereby resulting in an increase in myelinated nerve fibers in the saphenous nerve without changing DRG number. BDNF overexpression did not increase the number of myelinated nerve fibers in the saphenous nerve, suggesting that BDNF and NT4 function uniquely to influence neuron branching and morphologies of responsive DRG neurons. The differential effects of BDNF and NT4 on neuronal development have been observed in several sensory neuron populations. For example, the morphology of chorda tympani axons innervating taste buds is influenced by BDNF and NT4, but in very different manners. BDNF increases chorda tympani axon branching and promotes innervation of non-gustatory filiform papillae (Lopez and Krimm, 2003) whereas NT4 increases fiber fasciculation and inhibits branching. In retinal ganglion neurons, BDNF promotes branching and complexity of terminal axonal arbors, while NT4 has little influence on the morphological development of these cells (Cohen-Cory and Fraser, 1995). Finally, NT4 increases the length and complexity of basal dendrites in layer 4 cortical neurons, while BDNF influences the dendrites of layer 5 and 6 neurons (McAllister et al., 1995). The findings described here echo observations of BDNF and NT4’s divergent effects in other systems and extend them to the cutaneous system.
It should be mentioned that some of the observed differences between BDNF-OE and NT4-OE mice could be quantitative rather than qualitative. ELISA measurements of neurotrophin levels in dorsal back skin revealed a 4.3-fold increase in BDNF in BDNF-OE mice (LeMaster et al., 1999), while NT4 levels are increased by 2.3-fold in NT4-OE mice. It is possible that NT4 levels are sub-threshold for increasing glabrous Merkel cell number. On the other hand, since BDNF-OE mice express more robust levels of neurotrophin, the difference in neurotrophin levels cannot account for increased numbers of myelinated saphenous axons in NT4-OE mice compared to BDNF-OE mice. Therefore, while some of the differences described here may be due to quantitative differences in neurotrophin levels, some of the differences must be qualitative.
That BDNF and NT4 have overlapping and distinct effects suggests complex signaling regulation following trkB activation. Binding of BDNF to trkB may result in receptor configurations different than those following NT4 binding leading to the activation BDNF-specific intracellular signaling pathways. Consistent with this hypothesis, a point mutation of the Shc adapter binding site in trkB produced a phenotype similar to that found in mice lacking NT4 (Minichiello et al., 1998). Thus, NT4 signaling may be more dependent on Shc binding than BDNF, which is known to use a combination of binding sites (Minichiello et al., 2002; Medina et al., 2004). Alternative splicing of the trkB locus may also underlie differential activation by NT4 and BDNF. Full-length trkB and truncated trkB are known to differentially regulate dendritic morphologies of cortical neurons (Yacoubian and Lo, 2000).
In summary, NT4 overexpression increases sensory innervation to dermal papillae of the footpad, Meissner corpuscle size, and hair follicle innervation. Unlike BDNF overexpression, NT4 overexpression did not affect footpad Merkel cell number and innervation. As in BDNF-OE mice, NT4-OE mice displayed an increase in skin innervation without changing the number of sensory neurons. Also unlike BDNF-OE mice, myelinated nerve fibers within the saphenous nerve were increased as a result of NT4 overexpression. These results suggest that enhanced levels of NT4 modulate sensory neuron branching within the nerve in contrast to BDNF, which influences branching primarily in the skin.
Figure 5.
Overexpression of NT4 increases the proportion of TrkB expressing neurons but decreases the proportion of trkC expressing DRG neurons. The proportion of trkB labeled neurons in DRG of NT4-OE mice (B) was increased compared to wild type mice (A). The proportion of trkC labeled neurons in wild type DRGs (C) was decreased compared with DRGs of NT4-OE mice. Scale bar in D = 50µm and applies to all.
Acknowledgement
This work was supported by grants from the NIH to RFK (NIH DC04763) and KMA (NIH NS033730).
REFERENCES
- Airaksinen MS, Koltzenburg M, Lewin GR, Masu Y, Helbig C, Wolf E, Brem G, Toyka KV, Thoenen H, Meyer M. Specific subtypes of cutaneous mechanoreceptors require neurotrophin-3 following peripheral target innervation. Neuron. 1996;16:287–295. doi: 10.1016/s0896-6273(00)80047-1. [DOI] [PubMed] [Google Scholar]
- Albers KM, Perrone TN, Goodness TP, Jones ME, Green MA, Davis BM. Cutaneous overexpression of NT-3 increases sensory and sympathetic neuron number and enhances touch dome and hair follicle innervation. J Cell Biol. 1996;134:487–497. doi: 10.1083/jcb.134.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers KM, Wright DE, Davis BM. Overexpression of nerve growth factor in epidermis of transgenic mice causes hypertrophy of the peripheral nervous system. J Neurosci. 1994;14:1422–1432. doi: 10.1523/JNEUROSCI.14-03-01422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchkarev VA, Kief S, Paus R, Moll I. Overexpression of brain-derived neurotrophic factor increases Merkel cell number in murine skin. J Invest Dermatol. 1999;113:691–692. doi: 10.1046/j.1523-1747.1999.00725.x. [DOI] [PubMed] [Google Scholar]
- Cohen-Cory S, Fraser SE. Effects of brain-derived neurotrophic factor on optic axon branching and remodelling in vivo. Nature. 1995;378:192–196. doi: 10.1038/378192a0. [DOI] [PubMed] [Google Scholar]
- Conover JC, Erickson JT, Katz DM, Bianchi LM, Poueymirou WT, McClain J, Pan L, Helgren M, Ip NY, Boland P, et al. Neuronal deficits, not involving motor neurons, in mice lacking BDNF and/or NT4. Nature. 1995;375:235–238. doi: 10.1038/375235a0. [DOI] [PubMed] [Google Scholar]
- Davis BM, Fundin BT, Albers KM, Goodness TP, Cronk KM, Rice FL. Overexpression of nerve growth factor in skin causes preferential increases among innervation to specific sensory targets. J Comp Neurol. 1997;387:489–506. doi: 10.1002/(sici)1096-9861(19971103)387:4<489::aid-cne2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Fan G, Egles C, Sun Y, Minichiello L, Renger JJ, Klein R, Liu G, Jaenisch R. Knocking the NT4 gene into the BDNF locus rescues BDNF deficient mice and reveals distinct NT4 and BDNF activities. Nat Neurosci. 2000;3:350–357. doi: 10.1038/73921. [DOI] [PubMed] [Google Scholar]
- Figueiredo HF, Davis BM, Albers KM. Skin-derived nerve growth factor blocks programmed cell death in the trigeminal ganglia but does not enhance neuron proliferation. Mech Dev. 2001;109:205–214. doi: 10.1016/s0925-4773(01)00525-1. [DOI] [PubMed] [Google Scholar]
- Fundin BT, Silos-Santiago I, Ernfors P, Fagan AM, Aldskogius H, DeChiara TM, Phillips HS, Barbacid M, Yancopoulos GD, Rice FL. Differential dependency of cutaneous mechanoreceptors on neurotrophins, trk receptors, and P75 LNGFR. Dev Biol. 1997;190:94–116. doi: 10.1006/dbio.1997.8658. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Martinez T, Farinas I, Del Valle ME, Feito J, Germana G, Cobo J, Vega JA. BDNF, but not NT-4, is necessary for normal development of Meissner corpuscles. Neurosci Lett. 2005;377:12–15. doi: 10.1016/j.neulet.2004.11.078. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Martinez T, Germana GP, Monjil DF, Silos-Santiago I, de Carlos F, Germana G, Cobo J, Vega JA. Absence of Meissner corpuscles in the digital pads of mice lacking functional TrkB. Brain Res. 2004;1002:120–128. doi: 10.1016/j.brainres.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Harada S, Yamaguchi K, Kanemaru N, Kasahara Y. Maturation of taste buds on the soft palate of the postnatal rat. Physiol Behav. 2000;68:333–339. doi: 10.1016/s0031-9384(99)00184-5. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Matsuo S, Silos-Santiago I, Sugimoto T. Developmental dependency of Meissner corpuscles on trkB but not trkA or trkC. Neuroreport. 2000;11:259–262. doi: 10.1097/00001756-200002070-00007. [DOI] [PubMed] [Google Scholar]
- Jones KR, Farinas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkelin I, Stucky CL, Koltzenburg M. Postnatal loss of Merkel cells, but not of slowly adapting mechanoreceptors in mice lacking the neurotrophin receptor p75. Eur J Neurosci. 1999;11:3963–3969. doi: 10.1046/j.1460-9568.1999.00822.x. [DOI] [PubMed] [Google Scholar]
- Kitzman PH, Perrone TN, LeMaster AM, Davis BM, Albers KM. Level of p75 receptor expression in sensory ganglia is modulated by NGF level in the target tissue. J Neurobiol. 1998;35:258–270. doi: 10.1002/(sici)1097-4695(19980605)35:3<258::aid-neu3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Krimm RF, Davis BM, Albers KM. Cutaneous overexpression of neurotrophin-3 (NT3) selectively restores sensory innervation in NT3 gene knockout mice. J Neurobiol. 2000;43:40–49. doi: 10.1002/(sici)1097-4695(200004)43:1<40::aid-neu4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Krimm RF, Davis BM, Albers KM. Fungiform papillae and circumvallate taste buds are lost in mice lacking the neurotrophin receptor p75. Chemical Senses Abstract. 2001a [Google Scholar]
- Krimm RF, Davis BM, Woodbury CJ, Albers KM. NT3 expressed in skin causes enhancement of SA1 sensory neurons that leads to postnatal enhancement of Merkel cells. J Comp Neurol. 2004;471:352–360. doi: 10.1002/cne.20041. [DOI] [PubMed] [Google Scholar]
- Krimm RF, Miller KK, Kitzman PH, Davis BM, Albers KM. Epithelial overexpression of BDNF or NT4 disrupts targeting of taste neurons that innervate the anterior tongue. Dev Biol. 2001b;232:508–521. doi: 10.1006/dbio.2001.0190. [DOI] [PubMed] [Google Scholar]
- LeMaster AM, Krimm RF, Davis BM, Noel T, Forbes ME, Johnson JE, Albers KM. Overexpression of brain-derived neurotrophic factor enhances sensory innervation and selectively increases neuron number. J Neurosci. 1999;19:5919–5931. doi: 10.1523/JNEUROSCI.19-14-05919.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebl DJ, Klesse LJ, Tessarollo L, Wohlman T, Parada LF. Loss of brain-derived neurotrophic factor-dependent neural crest-derived sensory neurons in neurotrophin-4 mutant mice. Proc Natl Acad Sci U S A. 2000;97:2297–2302. doi: 10.1073/pnas.040562597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebl DJ, Tessarollo L, Palko ME, Parada LF. Absence of sensory neurons before target innervation in brain-derived neurotrophic factor-, neurotrophin 3-, and TrkC-deficient embryonic mice. J Neurosci. 1997;17:9113–9121. doi: 10.1523/JNEUROSCI.17-23-09113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ernfors P, Wu H, Jaenisch R. Sensory but not motor neuron deficits in mice lacking NT4 and BDNF. Nature. 1995;375:238–241. doi: 10.1038/375238a0. [DOI] [PubMed] [Google Scholar]
- Lopez GF, Krimm RF. Epithelial overexpression of BDNF and NT4 produce diverse gustatory axon morphologies. Neuroscience Abstracts. 2003 doi: 10.1016/j.ydbio.2006.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister AK, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- Medina DL, Sciarretta C, Calella AM, Von Bohlen Und Halbach O, Unsicker K, Minichiello L. TrkB regulates neocortex formation through the Shc/PLCgamma-mediated control of neuronal migration. Embo J. 2004;23:3803–3814. doi: 10.1038/sj.emboj.7600399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills LR, Nurse CA, Diamond J. The neural dependency of Merkel cell development in the rat: the touch domes and foot pads contrasted. Dev Biol. 1989;136:61–74. doi: 10.1016/0012-1606(89)90130-9. [DOI] [PubMed] [Google Scholar]
- Minichiello L, Calella AM, Medina DL, Bonhoeffer T, Klein R, Korte M. Mechanism of TrkB-mediated hippocampal long-term potentiation. Neuron. 2002;36:121–137. doi: 10.1016/s0896-6273(02)00942-x. [DOI] [PubMed] [Google Scholar]
- Minichiello L, Casagranda F, Tatche RS, Stucky CL, Postigo A, Lewin GR, Davies AM, Klein R. Point mutation in trkB causes loss of NT4-dependent neurons without major effects on diverse BDNF responses. Neuron. 1998;21:335–345. doi: 10.1016/s0896-6273(00)80543-7. [DOI] [PubMed] [Google Scholar]
- Moll I, Kuhn C, Moll R. Cytokeratin 20 is a general marker of cutaneous Merkel cells while certain neuronal proteins are absent. J Invest Dermatol. 1995;104:910–915. doi: 10.1111/1523-1747.ep12606183. [DOI] [PubMed] [Google Scholar]
- Rice FL, Albers KM, Davis BM, Silos-Santiago I, Wilkinson GA, LeMaster AM, Ernfors P, Smeyne RJ, Aldskogius H, Phillips HS, Barbacid M, DeChiara TM, Yancopoulos GD, Dunne CE, Fundin BT. Differential dependency of unmyelinated and A delta epidermal and upper dermal innervation on neurotrophins, trk receptors, and p75LNGFR. Dev Biol. 1998;198:57–81. [PubMed] [Google Scholar]
- Stucky CL, DeChiara T, Lindsay RM, Yancopoulos GD, Koltzenburg M. Neurotrophin 4 is required for the survival of a subclass of hair follicle receptors. J Neurosci. 1998;18:7040–7046. doi: 10.1523/JNEUROSCI.18-17-07040.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucky CL, Koltzenburg M, Schneider M, Engle MG, Albers KM, Davis BM. Overexpression of nerve growth factor in skin selectively affects the survival and functional properties of nociceptors. J Neurosci. 1999;19:8509–8516. doi: 10.1523/JNEUROSCI.19-19-08509.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeder V, Grim M, Kucera J, Sieber-Blum M. Neurotrophin-3 signaling in mammalian Merkel cell development. Dev Dyn. 2003;228:623–629. doi: 10.1002/dvdy.10403. [DOI] [PubMed] [Google Scholar]
- Vassar R, Fuchs E. Transgenic mice provide new insights into the role of TGF-alpha during epidermal development and differentiation. Genes Dev. 1991;5:714–727. doi: 10.1101/gad.5.5.714. [DOI] [PubMed] [Google Scholar]
- Wang X, Zinkel S, Polonsky K, Fuchs E. Transgenic studies with a keratin promoterdriven growth hormone transgene: prospects for gene therapy. Proc Natl Acad Sci U S A. 1997;94:219–226. doi: 10.1073/pnas.94.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoubian TA, Lo DC. Truncated and full-length TrkB receptors regulate distinct modes of dendritic growth. Nat Neurosci. 2000;3:342–349. doi: 10.1038/73911. [DOI] [PubMed] [Google Scholar]