Abstract
Gastrin is secreted from neuroendocrine cells residing in the adult antrum called G cells, but constitutively low levels are also expressed in the duodenum and fetal pancreas. Gastrin normally regulates gastric acid secretion by stimulating the proliferation of enterochromaffin-like cells and the release of histamine. Gastrin and progastrin forms are expressed in a number of pathological conditions and malignancies. However, the DNA regulatory elements in the human versus the mouse gastrin promoters differ suggesting differences in their transcriptional control. Thus, we describe here the expression of the human gastrin gene using a bacterial artificial chromosome (BAC) in the antral and duodenal cells of gastrin null mice. All 5 founder lines expressed the 253 kb human gastrin BAC. hGasBac transgenic mice were bred onto a gastrin null background so that the levels of human gastrin peptide could be analyzed by immunohistochemistry and radioimmunoassay without detecting endogenous mouse gastrin. We have shown previously that chronically elevated gastrin levels suppress somatostatin. Indeed, infusion of amidated rat gastrin depressed somatostatin levels, stimulated gastric acid secretion and an increase in the numbers of G cells in the antrum and duodenum. In conclusion, human gastrin was expressed in mouse enteroendocrine cells and was regulated by somatostatin. This mouse model provides a unique opportunity to study regulation of the human gastrin promoter in vivo by somatostatin and possibly other extracellular regulators contributing to our understanding of the mechanisms involved in transcriptional control of the human gene.
Keywords: somatostatin, BAC vector, gastrin transgene, duodenum, MEN 1
Introduction
Gastrin is an important gastrointestinal hormone secreted from neuroendocrine cells located in the antrum of the stomach and proximal duodenum (G cells) 1. However, only G cells in the gastric antrum are regulated by changes in gastric pH, fasting and re-feeding 2, 3. By contrast, gastrin levels produced by the few G cells in the adult duodenum are constitutively low 4. In addition to changes in gastric pH, chronic gastritis in human subjects increases gastrin gene expression, presumably due to reduced somatostatin expression 5 6, although the effect on G and D cell numbers is not consistently observed 7, 8 9. Nevertheless, we have shown previously in the somatostatin-deficient mouse that modulation of tissue somatostatin levels is the most effective regulator of gastrin secretion 10. In addition, proinflammatory cytokines, e.g., interferon gamma (IFNγ) and IL-1β, are effective regulators of somatostatin and subsequently gastrin 10, 11. However, the inducible and tissue-specific DNA regulatory elements controlling human gastrin gene expression have been difficult to study in vivo due to the lack of appropriate mouse models in which transgenic constructs are faithfully expressed in the antral and duodenal G cells.
Previous reports have described the use of gastrin promoters to drive the expression of human gastrin or other cDNAs in mice 12–14. By contrast, when a human promoter <3 kb was used to express the human gene in mice, no expression was detected 14. This result was likely due to insufficient amounts of the promoter in the transgene available to drive tissue-specific expression in mouse cells 14. When approximately 5 kb of the human gastrin promoter was used to drive SV40 T antigen expression, gastrin-expressing antral, islet and hepatobiliary tumors developed 13. The mice did not survive due to the tumor burden. This result supported the notion that tissue specific expression of the human promoter in the stomach requires at least 5 kb of upstream regulatory sequences, but that additional elements were required for the expression to be extinguished in the adult pancreas and liver. To date, none of the prior constructs completely recapitulate the native tissue specific expression pattern of human gastrin in the adult mouse.
Since there are human diseases, for example gastrinomas, in which the human gastrin promoter is overexpressed and mouse and human DNA regulatory elements differ significantly 15, 16, we propose that it will be important to study the human promoter directly. Human G cells are not readily available and genetic manipulation of the human promoter cannot be studied in vivo. Therefore, we generated a mouse model of human gastrin gene expression. To overcome most of the prior difficulties encountered when studying the human promoter in mice such as restriction of the expression to the adult stomach and duodenum, we used a human BAC clone 17, 18. Although prior mouse models of human gastrin gene expression were successful in targeting the antral G cell, none examined expression in the duodenum nor tested regulation of the human constructs in vivo 12–14.
In the current study, we describe a transgenic mouse that faithfully expresses human gastrin in both antral and duodenal G cells from a 253 kb human BAC clone containing the entire gastrin locus. Moreover, we demonstrate that modulating endogenous somatostatin levels regulate the human gastrin BAC transgene. Thus, this hGasBAC mouse model can be used to elucidate the transcriptional regulatory elements in the human gastrin promoter that function in vivo.
Materials and Methods
Animals
Wild-type (WT, C57BL/6) and gastrin-deficient (Gas−/− on a mixed 129/Sv/C57BL/6 genetic background) mice were maintained in individual sterile micro-isolator cages in non-barrier mouse rooms. All studies were performed with the approval of the University of Michigan Animal Care and Use Committee accredited by the American Association of Assessment and Accreditation of Laboratory Care (AAALAC) facility.
Human Gastrin BAC (hGasBAC) clone acquisition and characterization
The BAC clone RP11-1027A8, (GenBank Accession No. AC130341) was identified in silico as encompassing the human gastrin gene and was purchased from the Children’s Hospital of Oakland Research Institute (CHORI). The insert was 253 kb and was cloned into the pBACe3.6 vector (GenBank Accession No. U80929) 17. Since the available BAC DNA sequence available online was incomplete, we used sequence-tagged-site (STS)-content analysis and restriction mapping to assess the integrity of the cloned insert. The sequence-tagged-sites used for this analysis, the forward and reverse primers and amplimer sizes are shown in Table 1.
Table 1.
| Locus (STS) | FOR primer (5′-3′) | REV primer (5′-3′) | Amplimer size |
|---|---|---|---|
| D17S1210: | TGGAAATGCTGATGCAGCTA | TCGTGCAGTGACAAAGGTAT | 241 bp |
| SHGC-6077: | ATGTTATCACTTTGGAGACTGC | GTTTTCCCCAAACTTGATTC; | 199 bp |
| SHGC-12347: | GTCTATTGCAGGAGAAACGTCC | ATTTGCAAAAGGAAAAGTAATTCTT | 274 bp |
| SHGC-150918: | CTGATCTGAAGCCAATGACAAGT | GTCTGGCACATACATGAAGGCAT | 255 bp |
| SGC-35551: | CTCAGGCCTAGGAGGCC | AGATAATGAAGCTGTATTGATTGCC | 129 bp |
| STS-M15958: | CAGAGCCTCCGACCTTG | TTGACCCTGTGGTAGAGAATAAC | 175 bp |
| STS-W67907: | ATGAAGCATCCTTAGGCAGC | TTAAGTGTTGGGAGGCCATC | 151 bp |
| GDB:196353: | GAACACAGGAGTTTGAGTCC | TCGACTCTCCCTTCTCTCTC | 125 bp |
Generation of transgenic mice
Gastrin null mice were obtained from Linda Samuelson (University of Michigan) 19. ES cells from Gas−/− mice were injected with the linearized full-length human gastrin gene BAC vector. Blastocyts containing the 253 kb BAC clone were transplanted into pseudo-pregnant mothers. The offspring of these mice were bred to Gas−/− male mice on a congenic C57/BL6 background to maintain the hGasBAC transgene on a genetic background null for mouse gastrin. Human gastrin was detected by immunohistochemistry, radioimmunoassay and immunoblot analysis. The absence of mouse gastrin mRNA expression in the gastrin null mice was confirmed using mouse gastrin specific primers.
Administration of Gastrin-17 (G-17) to hGasBAC transgenic mice
Alzet micro-osmotic pumps (Model 1007D, Durect Corporation, Cupertino, CA) were inserted into the peritoneal cavity of hGasBAC or WT mice after anesthetizing with xylazine (20 mg/ml) and ketamine (100 mg/ml). The muscle was closed with sterile Ethicon dissolvable sutures and skin incisions were closed with nylon sutures. The pumps delivered PBS or rat gastrin G-17 (Bachem) at a rate of 5 μg/kg/h (30–40 μg/mouse/day of peptide) for 7 days before being sacrificed.
Immuohistochemistry
Human gastrin was detected by immunohistochemistry according to a previously published protocol 10. Briefly, longitudinal sections of the stomach antrum and duodenum were fixed in 4% paraformaldehyde/PBS then paraffin embedded. Three micron sections were prepared. The slides were de-paraffinized by heating to 100°C in 0.01 M sodium citrate for antigen retrieval and non-specific antigenic sites were blocked with 20% normal rabbit serum/PBS and 0.1% Triton X-100 for 30 min. The slides were incubated for 1 h with a 1:800 dilution of goat anti-gastrin (Santa Cruz Biotechnology). A 1:200 dilution of FITC-conjugated anti-goat (Jackson Laboratories) was used as the secondary antibody. The number of gastrin-stained cells was quantified by morphometry and expressed as the average number of cells counted per gland (10 glands counted per slide per animal). Stomach sections from WT and Gas−/− mice were used as positive and negative immunohistochemistry controls.
Gastrin radioimmunoassay (RIA)
RIA was used to determine the levels of gastrin in both tissue and serum of the hGasBAC mice. Approximately 1 ml of blood was collected by cardiac puncture, aliquoted into lithium-heparin tubes (SARSTEDT, Germany) then centrifuged at 3000 rpm for 15 min at 4°C to generate plasma (supernatant). The plasma was collected immediately and stored at −20°C until assayed for gastrin by RIA as previously described 20. Water tissue extracts were assayed for amidated gastrin (G-17) using the appropriate volume and dilution (empirically determined). Briefly, mouse serum (Sigma) was heat-inactivated at 56°C then charcoal stripped overnight at room temperature. For the standard curve, 1 mg/ml of human synthetic gastrin (Sigma) was diluted in PBS to 100 ng/ml, and then serially diluted in stripped mouse serum (Sigma) to: 1000, 512, 256, 128, 64, 32, 16, 8, and 4 pg/ml. The standard curve and test samples were performed in triplicate. 125I-G17 (5000 cpm per 50 ml PBS, Perkin Elmer LAS) competed for gastrin in the standards or samples for the binding of rabbit anti-gastrin antibody (1:5000 dilution, Dako Cytomation cat. # A0568), by incubating the reaction mixture for 72h at 4°C in tubes pre-coated with polyethylene glycol (2.5% MW 10000 Da from Sigma). The incubation period was terminated by adding 50 ml of newborn calf serum (Sigma) prior to precipitating bound gastrin with 250 ml of 25% polyethylene glycol (MW 8000 daltons, Sigma). After vortexing then centrifuging at 1500 rpm for 30 min at 4°C, the supernatant was removed by aspiration and the pellet counted on a gamma counter. The data was analyzed using non-linear regression analysis using Excel and Prism software to compute the levels of the gastrin in test samples from a standard curve.
Measurement of basal gastric acid levels
After sacrificing, WT, Gas−/−, hGasBAC and hGasBac mice infused with rat G-17, 2 ml of saline were used to rinse the stomach. The hydrogen ion concentration was determined by base titration using 0.005N NaOH and the pH-STAT control titrator (PHM290, Radiometer Analytical S.A. France). The concentration of acid was expressed as μEq of acid per kg weight of the mouse.
Immunoblot analysis
Protein was extracted from stomachs removed from WT, Gas−/− or hGasBac mice after homogenizing the tissue in lysis buffer (300 mmol/L NaCl, 30 mmol/L Tris, 2 mmol/L MgCl2, 2 mmol/L CaCl2, 1% Triton X-100, pH 7.4) supplemented with protease inhibitors (EDTA-free complete tablets, Roche). Immunoblot analysis was performed on a 4–20% SDS-polyacrylamide gradient gel (Invitrogen, Carlsbad, CA, USA). The nylon membranes were blocked with 0.55 Uniblock (Analytical Genetic Testing Center, Inc.) for 1h at room temperature prior to a1h incubation with goat anti-gastrin antibody (Santa Cruz) at a dilution of 1:1000. The membranes were washed three times for 10 min followed by incubating with a 1:2000 dilution of Alexa Fluor 680 rabbit anti-goat (Molecular Probes) for an additional hour. Gastrin pro-peptide was visualized using the Odyssey Infrared Imaging System (Li-Cor Biosciences, Lincoln, NE). All immunoblots were quantified using the Odyssey Infrared Imaging System software and the data was expressed in pixels/mm2.
PCR analysis
cDNAs were reverse transcribed from total RNA obtained from WT, Gas−/− and hGasBAC stomachs using an oligo-dT primer according to the manufacturer’s protocol (GIBCO-BRL, Superscript kit). To confirm the integrity of the RNA prepared, the cDNAs were also subjected to PCR amplification of GAPDH. All primers were obtained from Invitrogen. The forward primer sequences for human gastrin was: ATGCAGCGACTATGTGTGTGTATG and the reverse primer was: TTCTCATCCTCAGCACTGCGGCGGC. The size of the gastrin fragment was 432 bp. For GAPDH, the forward primer was: TTCACCACCATGGAGAAGGC, and the reverse primer was: GGCATGGACTGTGGTCATGA. The amplified GAPDH product was 612 bp. PCR amplification was performed in a total volume of 25 μl, containing 105 PCR buffer with MgCl2, 10nM dNTPs, 200nM of primers, 5 μl of cDNA, 100 nM of Taq Polymerase GOLD and 2.5 μl of Sybr Green (Molecular Probes). PCR amplification was performed in duplicate using the following conditions: 94°C for 10 min, followed by 35 two-temperature cycles at 94°C for 1 min and 55°C for 1 min.
Results
Characterization of the hGasBAC clone
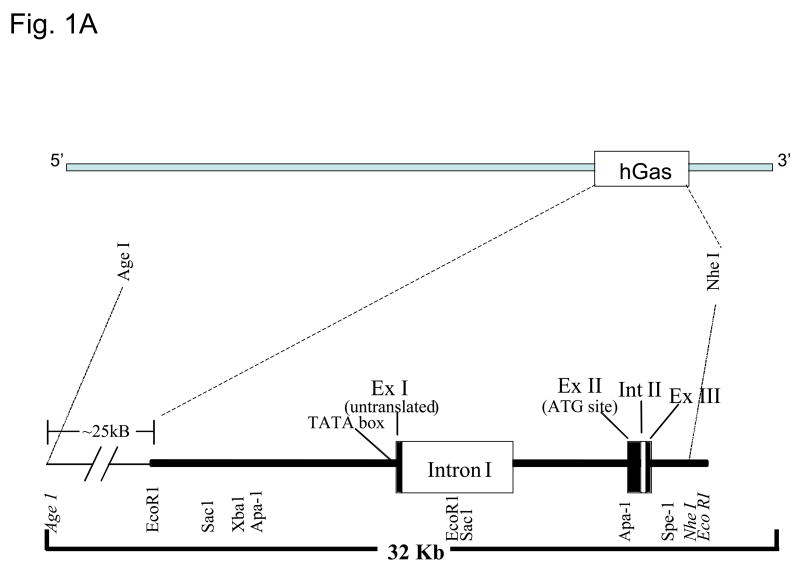
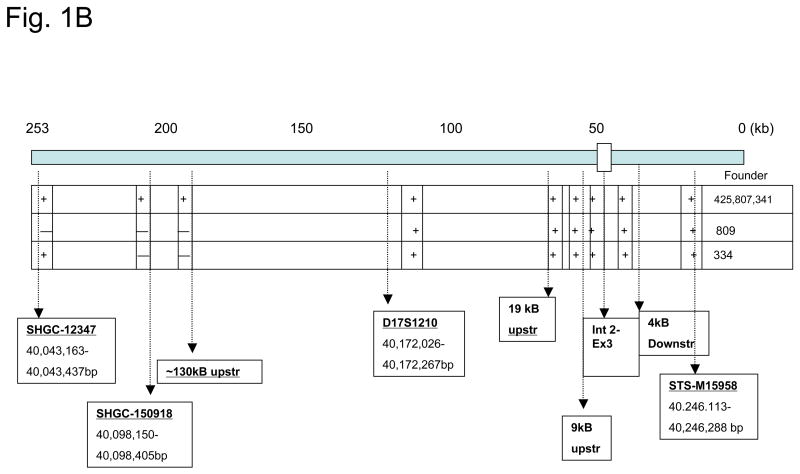
Since there were gaps in the DNA sequence of the 253 kb human gastrin BAC (hGasBAC), we performed sequence-tagged-site (STS)-content analysis, additional DNA sequencing and restriction mapping to confirm its structural integrity. The combined results of these analyses are summarized in Figure 1. The hBAC clone contained a contiguous insert spanning about 253 kb (Fig. 1A), with the gastrin gene locus near the 3′ end of the clone (~50 kb from the 3′ terminus). The gastrin insert with the sequence-tagged-sites and annotated restriction map for the human gastrin locus is shown in Fig. 1B.
Figure 1. Characterization of hGasBAC.
Panel A. The genomic segment encompassing the human gastrin gene locus is represented as a horizontal line. Exons are represented as filled boxes and introns are unshaded. The various restriction sites spanning the 5′ and 3′ regions and the size of the genomic human gastrin gene hGas is indicated.
Panel B. Long-range map showing the various sequence-tagged-site (STS) and restriction sites for clone characterization of the genomic segment.
Transgenic hGASBAC mice express human gastrin
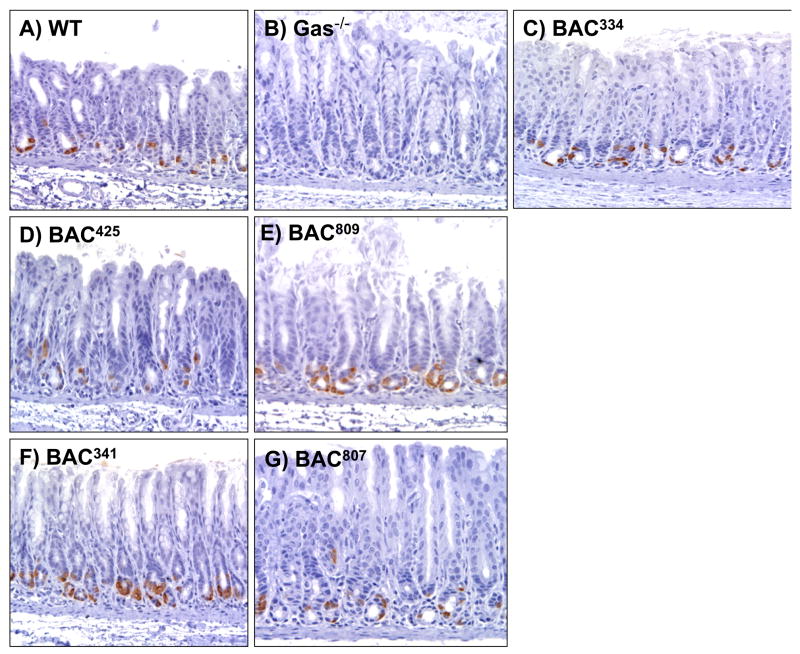
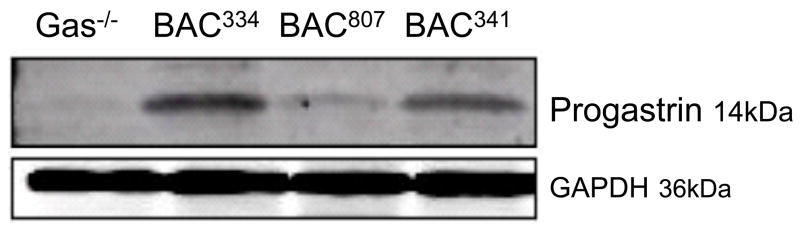
The hGasBAC transgenic mice were bred onto the gastrin null (Gas−/−) background so as to analyze the expression and phenotype of the mice exclusively expressing human gastrin without interference from endogenous mouse gastrin. Five founder lines of mice were analyzed and all were observed to express human gastrin in the antrum and duodenum. However, the amount of gastrin expressed based upon the intensity of gastrin detected by immunohistochemistry varied for each founder (Fig. 2). Founder lines 425 and 807 exhibited low levels of antral gastrin staining while 334, 809 and 341 showed high levels staining. We observed that all the mice expressed human gastrin predominately at the base of the antral glands as expected. The variation in gastrin peptide levels was confirmed by immunoblot analysis (Fig. 3) and tissue gastrin concentration determined by RIA (Fig. 4A). There was no gastrin expressed in the adult pancreas (data not shown). The variation in expression was likely due to differences in the site of transgene integration rather than stability of inheritance of the transgene since the analysis was carried out on the F2 generation of mice (two generations after the founder line). Nevertheless, it is worth noting that despite the degree of peptide expression, the transgene faithfully expressed in the correct tissues (antrum and duodenum and not the adult pancreas), suggesting that the additional sequences in the BAC served to extinguish inappropriate expression in other tissues and to insulate the transgene from spurious influences of the surrounding sequences at the random sites of integration 21–23.
Figure 2. Expression of human gastrin in the antrums of 5 founder lines.
Immunohistochemical staining for gastrin in the antrum of A) WT (wildtype), B) GAS−/−, and BAC mice from founder lines C) 334 (BAC334), D) 425 (BAC425), E) 809 (BAC809), F) 341 (BAC341) and G) 807 (BAC807).
Figure 3. Detection of the human gastrin in transgenic mice by immunoblot analysis.
Antral tissue collected from 3 hGas Bac mice was analyzed by immunoblot analysis using an antibody that recognizes unprocessed and processed gastrin (Santa Cruz) at 1:1000 dilution. Shown is the precursor progastrin (ProGAS) form detected at 14 kDa.
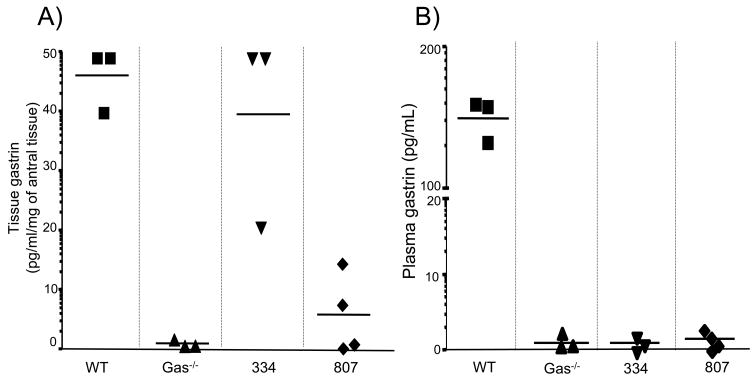
Figure 4. Detection of human gastrin in antral tissue by RIA.
A. Antral tissue levels of gastrin measured by RIA in hGasBAC founder lines 334 and 807 and compared to wild-type age-matched (controls) and gastrin knock-out mice (Gas−/−) as positive and negative controls, respectively.
B. Plasma gastrin concentrations in hGasBAC mouse lines 334 and 807 were compared to wild-type age-matched (controls) and gastrin knock-out mice (Gas−/−).
Human gastrin peptide detected in tissue not plasma
RIAs using a monoclonal antibody specific for amidated gastrin were used to quantify the amount of human gastrin expressed in the antral tissue of these hGasBAC transgenic mice. The amount and site of human gastrin transgene expression in line 334 was comparable to the amount of endogenous mouse gastrin quantified in the antral tissue of WT mice (40–45 pg/ml/mg tissue) (Fig. 4A). Founder line 809 expressed significantly lower levels of gastrin at a mean tissue concentration of 8 pg/ml/mg tissue consistent with the immunohistochemical staining. Thus, founder line 334 expressed high levels of gastrin, whereas founder line 809 expressed low levels of gastrin.
In contrast, gastrin peptide was not detected in the plasma of any of the hGasBAC mouse lines suggesting that although human gastrin is expressed and processed (antibody specific for amidated gastrin) in the tissue, it was not secreted. This result was not a function of the ability of the antibody to recognize gastrin from different species. Both plasma and tissue samples were assayed in the together. Therefore we concluded that the RIA quantified both plasma and tissue levels of gastrin in the WT mice but only tissue gastrin and not plasma gastrin were detected in the mice expressing the human transgene. Since the antibody detects only amidated gastrin, we concluded that the human transgene is expressed and the peptide is processed but not secreted from the cell. This is the first report of a species-specific difference in the gastrin gene sequence that translates into a functional distinction, i.e., gastrin secretion. The result suggests that there might be regulatory mechanisms related to the export of gastrin from the cell that have heretofore not been observed.
Acid and somatostatin regulate the human gastrin transgene
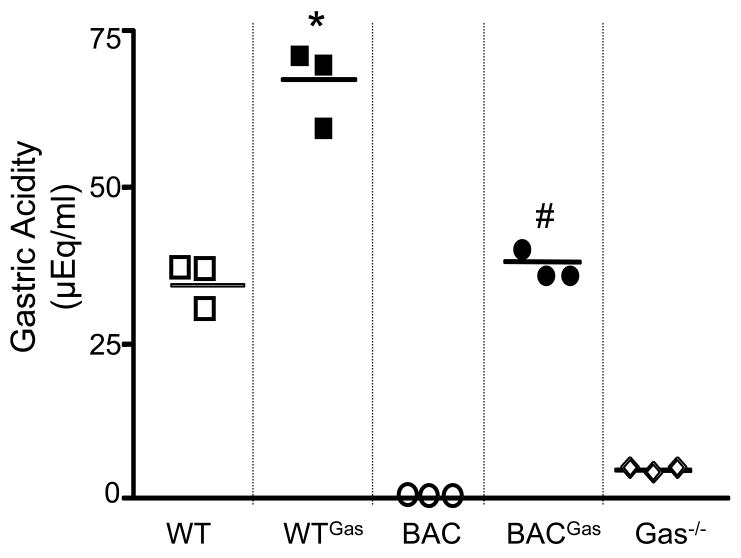
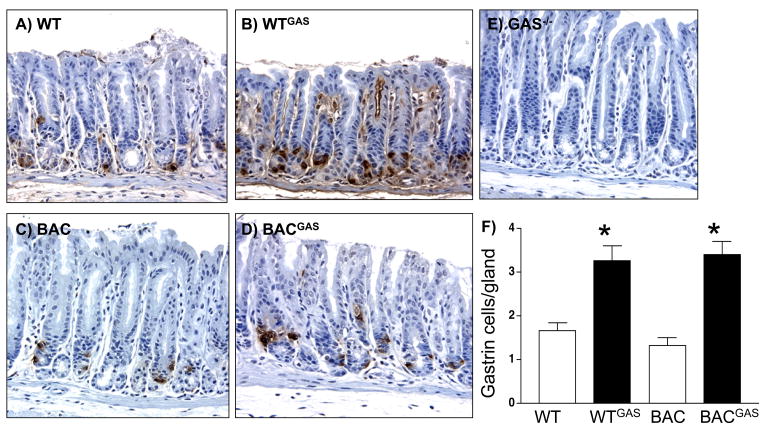
It has been established that the most effective means of stimulating gastrin physiologically is by suppressing somatostatin levels to remove its paracrine regulation of G-cells 24–26. Infusing exogenous gastrin acutely initially stimulates somatostatin release 27. However, we have found that chronic hypergastrinemia suppresses somatostatin 20. In order to determine whether the human gastrin BAC transgene expressed in these mice could be regulated, we infused a mixture of pro- and amidated rat gastrin G-17 into either the hGasBAC or wild type (WT) mice. We found that the infusion stimulated gastric acid secretion in the stomachs of both the WT and hGasBAC mice (line 807) on the gastrin-deficient background (Fig. 5). Moreover, the infusion essentially raised the acid levels in the transgenic mice to the levels produced in WT mice at baseline.
Figure 5. Gastric acidity is increased in hGas Bac mice infused with gastrin.
Gastric acidity in WT (wild type), gastrin infused WT (WTGAS), hGasBAC (BAC), infused with rodent gastrin BAC (BACGAS) and gastrin-deficient (GAS−/−) mice. *P<0.05 compared to WT, #P<0.05 compared to BAC. The results are expressed as the mean ± SEM for 3 mice per group (unpaired t-test).
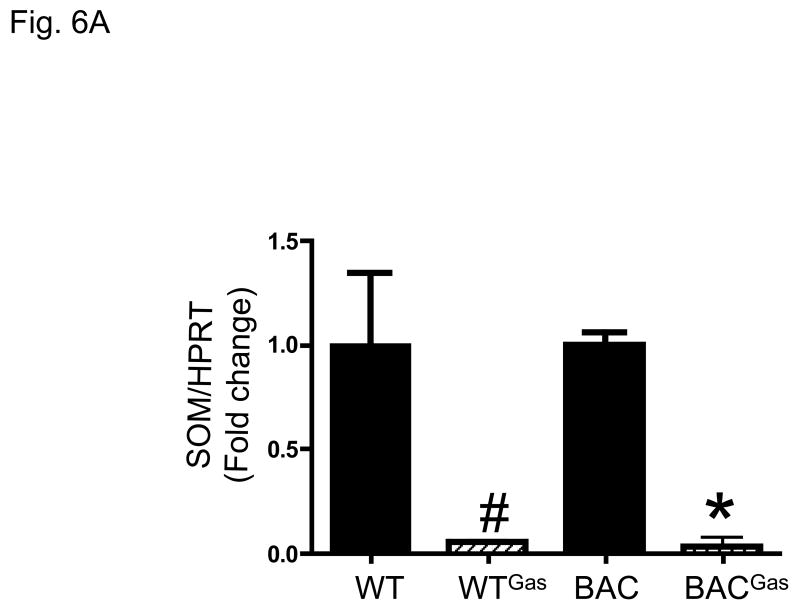
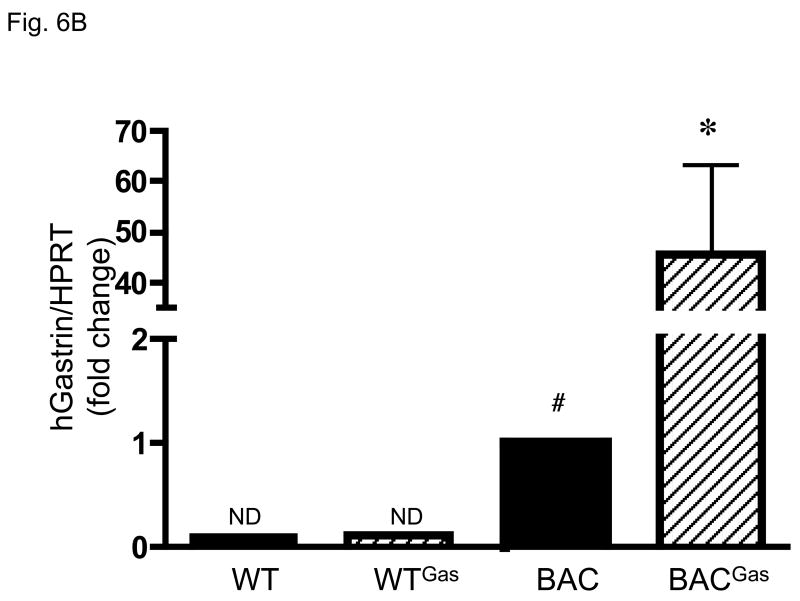
Previous data has shown that even in gastrin null mice, somatostain levels remain unchanged compared to WT 20. However, infusion of G-17 over 14 days was sufficient to decrease gastrin levels. Indeed, we confirmed that infusion of gastrin into WT or hGasBAC transgenic mice significantly depressed somatostatin mRNA levels (Fig. 6A). Using specific primers for exon 3 of the human gastrin gene that do not amplify mouse gastrin, we found that the decrease in somatostatin correlated with a significant increase in the mRNA levels of human gastrin (Fig. 6B). It should be emphasized that exon 3 human gastrin primers did not amplify mouse gastrin mRNA, explaining the result shown for the WT mice (Fig. 6B). Immunohistochemical staining with antibodies specific for amidated gastrin demonstrated increased gastrin expression in the antral tissues of both the hGasBAC and WT mice (Fig. 7), demonstrating that somatostatin regulates the expression of the human gastrin transgene. It is unclear why the peptide is not secreted from the mouse cells. Nevertheless, the result shows that somatostatin regulation of gastrin gene expression is not linked to gastrin secretion.
Figure 6. Hyperacidity decreases somatostatin levels in hGasBAC mice.
A. qRT-PCR levels for somatostatin on WT and hGasBAC mice infused with rat gastrin. *P<0.05 compared to BAC; #P<0.05 compared to WT; n = 3 per group (unpaired t-test).
B: Decrease in somatostatin modulates expression of human gastrin in hGasBAC mice. qRT-PCR levels for human gastrin on hGasBAC mice infused with rat gastrin. *P<0.05 compared to BAC; #P<0.05 compared to WT; n = 3 per group. ND: not detected. The PCR primers used in the analysis were specific for human and not mouse gastrin. Therefore no mouse gastrin mRNA was detected serving as a useful control analysis of expression of the human gastrin BAC mRNA.
Figure 7. Up-regulation of gastrin cells in the antrum of gastrin infused WT and hGasBAC mice.
A. Immunohistochemical staining for gastrin in antrums of A) WT (wild type), WT mice infused with rodent gastrin B) WT (WTGAS), C) hGas BAC mice (BAC), D) gastrin infused BAC (BACGAS) and E) gastrin-deficient (Gas−/−) mice. B. Quantification of the number of gastrin positive cells per gland was determined by morphometry. *P<0.05 compared to WT. The results are expressed as the mean ± SEM for 3 mice per group (unpaired t-test).
Expression and regulation of human gastrin in the duodenum of hGasBAC mice
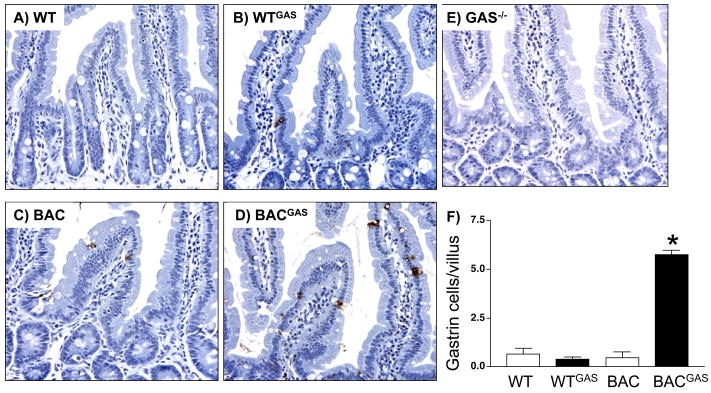
Gastrinomas developing in Men 1 patients are located primarily in the duodenum rather than the antrum or pancreas 28. This observation raises the possibility that regulation of gastrin gene expression in the duodenum differs from regulation in the stomach. In fact, duodenal enteroendocrine cells appear to be distinct from enteroendocrine cells in the stomach based on lineage tracing experiments using a neurogenin 3 homeobox promoter driving CRE recombinase 29. In the duodenum, we found that the induction of gastrin was much greater than in the antrums (compare Fig. 7 and 8). This result suggested that human gastrin in the duodenum is more sensitive to changes in somatostatin levels, since the levels were significantly increased upon infusion with exogenous gastrin while mouse duodenal gastrin was not.
Figure 8. Up-regulation of gastrin cells in the duodenum of gastrin infused hGasBAC mice.
A. Immunohistochemical staining for gastrin in duodenums of A) WT (wild type), gastrin infused B) WT (WTGAS), C) BAC, D) gastrin infused hGasBAC (BACGAS) and E) gastrin-deficient (Gas−/−) mice. Duodenal G cells indicated (arrow). B. Quantification of the number of gastrin positive cells per villus was determined by morphometry. *P<0.05 compared to WT. The results are expressed as the mean ± SEM for 3 mice per group (unpaired t-test).
Discussion
Gastrin has been implicated in a number of human disease states, e.g., chronic gastritis and gastrointestinal cancers 30–33. Presumably this is due to aberrant regulation of gastrin gene expression. Since proximal regulatory elements in the mouse and human promoters differ 15, 16, an in vivo model of human gastrin expression would be useful in furthering our understanding of how the human locus is regulated.
Previous reports have described appropriate tissue specific expression of the human gastrin coding sequences in G cells when expressed from the rat gastrin promoter 14. By contrast, studies using short segments of the human gastrin promoter were unsuccessful in driving expression 14. This result might be due to nucleotide differences within essential regulatory elements that affect the way transcription factors bind to the rodent versus the human gastrin promoters 15, 16. While reports using small fragments of the human gastrin gene (~1300 bp) have not been successful in driving expression 14, a 10.5 kB human gastrin construct comprised of ~5 kb of the human promoter in addition to the 3 exons, 2 introns and 1.5 kb of 3′ untranslated sequence linked to the SV40 T antigen coding sequence resulted in antral tumors expressing gastrin as well as islet and hepatobiliary tumors 13. Taken together, these studies suggest that the differences in tissue specific expression of gastrin reside within the upstream promoter elements. In particular, it appears that appropriate expression of the human promoter in mouse cells requires a large segment of the promoter.
Therefore to study regulation of the human gene in vivo, a transgenic mouse was generated from a human BAC clone. We successfully generated 5 lines of transgenic mice that expressed the human gastrin transgene on a gastrin null background. Interestingly, these hGasBAC mice remained hypochlorhydric and hypogastrinemic despite appropriate tissue-restricted gastrin expression in antral and duodenal tissues. We excluded differences in gastrin processing as the cause of the hypogastrinemia since the antibody used for immunohistochemical analysis and RIA detects only the amidated forms of gastrin. Furthermore since Wang and others 14 have previously shown that human gastrin expressed from the insulin promoter in the INS-GAS mouse model stimulates acid, we also excluded the possibility that mouse parietal cells are unresponsive to human gastrin peptides. The absence of gastrin in the serum suggests that the human gastrin locus contains DNA sequences that encode protein domains that are poorly conserved in the mouse locus and are ultimately required to export the peptide out of the neuroendocrine cell.
We also showed that modulating the levels of somatostatin regulated gastrin gene expression suggesting that the transcriptional control elements in the human gastrin BAC transgene are intact. We previously reported that exogenous gastrin infusion suppresses somatostatin levels and increases gastric acidity 20, supporting the observation that exogenous gastrin regulated the number of G-cells expressing the human peptide in the hGasBAC mice. Although this model may not be useful to study the effects of human gastrin in plasma on downstream targets, it remains a relevant model to study regulation of gene expression. An additional application of the human gastrin BAC transgene will be its ability to drive CRE recombinase to generate G cell specific knockout mice.
The difference in the regulation of the human gene in the duodenum compared to the mouse locus was not expected. However, further deletional analysis of the human BAC transgene may provide clues as to why gastrinomas develop in the duodenums of MEN 1 patients compared to the behavior of the mouse gastrin locus in mice heterozygous for the menin allele. Specifically, Collins and co-workers found that only 1 tumor developed in the duodenum of a mouse model of MEN 1 gastrinomas at 18 months 34. Although the delay in tumor development was thought to be a function of the time to develop loss of heterozygosity at the second locus, their result might also reflect species differences at the gastrin locus. In particular, the human gastrin gene might be more susceptible to mutations at the menin locus. In point of fact, we clearly showed here that the human gastrin gene is more responsive to regulation by somatostatin than the mouse locus.
In conclusion, the studies here describe the creation of a mouse model that expresses the human gastrin gene. The factors involved in secretion of the human gastrin peptide and transcriptional control of the human locus by at least one extracellular regulator, i.e., somatostatin, appear to be distinct compared to the mouse gene. We propose that further study of this transgenic hGasBac mouse model will be useful to study human gastrin regulatory elements in vivo and can also be used as a tool to express genes of interest in a bona fide G cell as opposed to tumor-derived cell lines or immortalized primary cells.
Acknowledgments
This work was supported in part by Public Health Service Grant R01-DK45729 (J.L.M.)
Abbreviations
- BAC
bacterial artificial chromosome
- hgastrin
human gastrin peptide
- MEN1
multiple endocrine neoplasia type 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schubert ML. Gastric secretion. Curr Opin Gastroenterol. 2007;23:595–601. doi: 10.1097/MOG.0b013e3282f03462. [DOI] [PubMed] [Google Scholar]
- 2.Brand SJ, Stone D. Reciprocal regulation of antral gastrin and somatostatin gene expression by omeprazole-induced achlorhydria. J Clin Invest. 1988;82:1059–1066. doi: 10.1172/JCI113662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu SV, Sumii K, Tari A, Sumii M, Walsh JH. Regulation of rat antral gastrin and somatostatin gene expression during starvation and after refeeding. Gastroenterology. 1991;101:1552–1558. doi: 10.1016/0016-5085(91)90391-w. [DOI] [PubMed] [Google Scholar]
- 4.Brand SJ, Fuller PJ. Differential gastrin gene expression in rat gastrointestinal tract and pancreas during neonatal development. J Biol Chem. 1988;263:5341–5347. [PubMed] [Google Scholar]
- 5.Sumii M, Sumii K, Tari A, Kawaguchi H, Yamamoto G, Takehara Y, Fukino Y, Kamiyasu T, Hamada M, Tsuda T, Yoshihara M, Haruma K, Kajiyama G. Expression of antral gastrin and somatostatin mRNA in Helicobacter pylori-infected subjects, Am. J Gastroenterol. 1994;89:1515–1519. [PubMed] [Google Scholar]
- 6.Park SM, Lee HR, Kim JG, Park JW, Jung G, Han SH, Cho JH, Kim MK. Effect of Helicobacter pylori infection on antral gastrin and somatostatin cells and on serum gastrin concentrations. Korean J Intern Med. 1999;14:15–20. doi: 10.3904/kjim.1999.14.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graham DY, Lew GM, Lechago J. Antral G-cell and D-cell numbers in Helicobacter pylori infection: Effect of H. pylori eradication. Gastroenterology. 1993;104:1655–1660. doi: 10.1016/0016-5085(93)90642-p. [DOI] [PubMed] [Google Scholar]
- 8.Chamouard P, Walter P, Wittersheim C, Demuynck P, Meunier O, Baumann R. Antral and fundic D-cell numbers in Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 1997;9:361–365. doi: 10.1097/00042737-199704000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Dial EJ, Hall LR, Romero JJ, Lechago J, Fox JG, Lichtenberger LM. Altered gastrin regulation in mice infected with Helicobacter felis. Dig Dis Sci. 2000;45:1308–1314. doi: 10.1023/a:1005543701212. [DOI] [PubMed] [Google Scholar]
- 10.Zavros Y, Rathinavelu S, Kao JY, Todisco A, DelValle J, Weinstock JV, Low MJJLM. Treatment of Helicobacter Gastritis With Interleukin-4 Requires Somatostatin. Proc Natl Acad Sci. 2003;100:12944–12949. doi: 10.1073/pnas.2135193100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang M, Furuta T, Takashima M, Futami H, Shirai N, Hanai H, Kaneko E. Relation between interleukin-1beta messenger RNA in gastric fundic mucosa and gastric juice pH in patients infected with Helicobacter pylori. J Gastroenterol. 1999;34:10–17. [PubMed] [Google Scholar]
- 12.Zhukova E, Afshar A, Ko J, Popper P, Pham T, Sternini C, Walsh JH. Expression of the human insulin gene in the gastric G cells of transgenic mice. Transgenic Res. 2001;10:329–341. doi: 10.1023/a:1016641530206. [DOI] [PubMed] [Google Scholar]
- 13.Montag AG, Oka T, Baek KH, Choi CS, Jay G, Agarwal K. Tumors in hepatobiliary tract and pancreatic islet tissues of transgenic mice harboring gastrin simian virus 40 large tumor antigen fusion gene. Proc Natl Acad Sci USA. 1993;90:6696–6700. doi: 10.1073/pnas.90.14.6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang TC, Babyatsky MW, Oates PS, Zhang Z, Tillotson L, Chulak M, Brand SJ, Schmidt EV. A rat gastrin-human gastrin chimeric transgene directs antral G cell-specific expression in transgenic mice. Am J Physiol. 1995;268:G1025–1036. doi: 10.1152/ajpgi.1995.268.6.G1025. [DOI] [PubMed] [Google Scholar]
- 15.Koh TJ, Wang TC. Molecular cloning and sequencing of the murine gastrin gene. Biochem Biophys Res Commun. 1995;216:34–41. doi: 10.1006/bbrc.1995.2588. [DOI] [PubMed] [Google Scholar]
- 16.Bundgaard JR, Hansen TO, Friis-Hansen L, Rourke IJ, van Solinge WW, Nielsen FC, Rehfeld JF. A distal Sp1-element is necessary for maximal activity of the human gastrin gene promoter. FEBS Lett. 1995;369:225–228. doi: 10.1016/0014-5793(95)00754-w. [DOI] [PubMed] [Google Scholar]
- 17.Frengen E, Weichenhan D, Zhao B, Osoegawa K, van Geel M, de Jong PJ. A modular, positive selection bacterial artificial chromosome vector with multiple cloning sites. Genomics. 1999;58:250–253. doi: 10.1006/geno.1998.5693. [DOI] [PubMed] [Google Scholar]
- 18.Jeong Y, Epstein DJ. Modification and production of BAC transgenes. Curr Protoc Mol Biol. 2005;Chapter 23:Unit 23 11. doi: 10.1002/0471142727.mb2311s71. [DOI] [PubMed] [Google Scholar]
- 19.Friis-Hansen L, Sundler F, Li Y, Gillespie PJ, Saunders TL, Greenson JK, Owyang C, Rehfeld JF, Samuelson LC. Impaired gastric acid secretion in gastrin-deficient mice. Am J Physiol. 1998;274:G561–568. doi: 10.1152/ajpgi.1998.274.3.G561. [DOI] [PubMed] [Google Scholar]
- 20.Zavros Y, Rieder G, Ferguson A, Samuelson LC, Merchant JL. Hypergastrinemia in response to gastric inflammation suppresses somatostatin. Am J Physiol Gastrointest Liver Physiol. 2002;282:G175–183. doi: 10.1152/ajpgi.00287.2001. [DOI] [PubMed] [Google Scholar]
- 21.Bao L, Zhou M, Cui Y. CTCFBSDB: a CTCF-binding site database for characterization of vertebrate genomic insulators. Nucleic Acids Res. 2008;36:D83–87. doi: 10.1093/nar/gkm875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filippova GN. Genetics and epigenetics of the multifunctional protein CTCF. Curr Top Dev Biol. 2008;80:337–360. doi: 10.1016/S0070-2153(07)80009-3. [DOI] [PubMed] [Google Scholar]
- 23.Valenzuela L, Kamakaka RT. Chromatin insulators. Annu Rev Genet. 2006;40:107–138. doi: 10.1146/annurev.genet.39.073003.113546. [DOI] [PubMed] [Google Scholar]
- 24.Saffouri B, Weir G, Bitar K, Makhlouf G. Stimulation of gastrin secretion from the perfused rat stomach by somatostatin antiserum. Life Sci. 1979;25:1749–1753. doi: 10.1016/0024-3205(79)90478-8. [DOI] [PubMed] [Google Scholar]
- 25.Schubert ML, Makhlouf GM. Neural, hormonal, and paracrine regulation of gastrin and acid secretion. Yale J Biol Med. 1992;65:553–560. [PMC free article] [PubMed] [Google Scholar]
- 26.Schubert ML, Makhlouf GM. In: The somatostatin cell as a local modulator of gastric function. Håkanson R, Sundler F, editors. Amsterdam: Elsevier; 1991. pp. 99–110. [Google Scholar]
- 27.Schubert ML, Saffouri B, Makhlouf GM. Identical patterns of somatostatin secretion from isolated antrum and fundus of rat stomach. Am J Physiol. 1988;254:G20–G24. doi: 10.1152/ajpgi.1988.254.1.G20. [DOI] [PubMed] [Google Scholar]
- 28.Anlauf M, Perren A, Meyer CL, Schmid S, Saremaslani P, Kruse ML, Weihe E, Komminoth P, Heitz PU, Kloppel G. Precursor lesions in patients with multiple endocrine neoplasia type 1-associated duodenal gastrinomas. Gastroenterology. 2005;128:1187–1198. doi: 10.1053/j.gastro.2005.01.058. [DOI] [PubMed] [Google Scholar]
- 29.Schonhoff SE, Giel-Moloney M, Leiter AB. Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev Biol. 2004;270:443–454. doi: 10.1016/j.ydbio.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Rindi G, Solcia E. Endocrine hyperplasia and dysplasia in the pathogenesis of gastrointestinal and pancreatic endocrine tumors. Gastroenterol Clin North Am. 2007;36:851–865. vi. doi: 10.1016/j.gtc.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Grabowska AM, Watson SA. Role of gastrin peptides in carcinogenesis. Cancer Lett. 2007;257:1–15. doi: 10.1016/j.canlet.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 32.Orlando LA, Lenard L, Orlando RC. Chronic hypergastrinemia: causes and consequences. Dig Dis Sci. 2007;52:2482–2489. doi: 10.1007/s10620-006-9419-3. [DOI] [PubMed] [Google Scholar]
- 33.Friis-Hansen L. Achlorhydria is associated with gastric microbial overgrowth and development of cancer: lessons learned from the gastrin knockout mouse. Scand J Clin Lab Invest. 2006;66:607–621. doi: 10.1080/00365510600873894. [DOI] [PubMed] [Google Scholar]
- 34.Crabtree JS, Scacheri PC, Ward JM, Garrett-Beal L, Emmert-Buck MR, Edgemon KA, Lorang D, Libutti SK, Chandrasekharappa SC, Marx SJ, Spiegel AM, Collins FS. A mouse model of multiple endocrine neoplasia, type 1, develops multiple endocrine tumors. Proc Natl Acad Sci U S A. 2001;98:1118–1123. doi: 10.1073/pnas.98.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]