Abstract
Hormonal activation of Gs, the stimulatory regulator of adenylyl cyclase, promotes dissociation of αs from Gβγ, accelerates removal of covalently attached palmitate from the Gα subunit, and triggers release of a fraction of αs from the plasma membrane into the cytosol. To elucidate relations among these three events, we assessed biochemical effects in vitro of attached palmitate on recombinant αs prepared from Sf9 cells. In comparison to the unpalmitoylated protein (obtained from cytosol of Sf9 cells, treated with a palmitoyl esterase, or expressed as a mutant protein lacking the site for palmitoylation), palmitoylated αs (from Sf9 membranes, 50% palmitoylated) was more hydrophobic, as indicated by partitioning into TX-114, and bound βγ with 5-fold higher affinity. βγ protected GDP-bound αs, but not αs· GTP[γS], from depalmitoylation by a recombinant esterase. We conclude that βγ binding and palmitoylation reciprocally potentiate each other in promoting membrane attachment of αs and that dissociation of αs·GTP from βγ is likely to mediate receptor-induced αs depalmitoylation and translocation of the protein to cytosol in intact cells.
Keywords: trimeric G proteins, lipid modification, membrane localization, protein–protein interaction
Heterotrimeric (αβγ) G proteins act as molecular switches to relay information from activated receptors to appropriate effector proteins (1, 2). Receptor-catalyzed replacement of bound GDP by GTP and GTP hydrolysis by the Gα subunit respectively turn on and turn off G protein-mediated signals. Gα·GDP binds to the βγ subunit and is inactive, while Gα·GTP dissociates from Gβγ, allowing both subunits to regulate effectors.
Efficient signaling requires location of G protein subunits at the cytoplasmic face of the plasma membrane, where they can interact with receptors and effectors. Three classes of lipid modification mediate and regulate locations of G protein subunits at the plasma membrane (3–5). G protein α subunits are covalently modified at or near their N termini by the fatty acids myristate and/or palmitate, while γ subunits are prenylated at their C termini. While myristoylation and isoprenylation are irreversible modifications, palmitoylation is reversible and subject to dynamic regulation in intact cells (3). The α subunit (αs) of Gs, the stimulatory regulator of adenylyl cyclase, is palmitoylated but not myristoylated (6). We have identified an αs palmitoylation cycle in intact cells (3, 7), in which hormone receptors appear to regulate membrane vs. cytosolic localization of αs by controlling its depalmitoylation by cellular esterase(s). Two sets of observations support this interpretation. An αs mutation (αs-C3S) replacing the amino acid attachment site of palmitate prevented membrane localization of αs and abrogated its ability to mediate hormonal regulation of adenylyl cyclase (8). In addition, Gs activation by stimulation of β-adrenoreceptors promoted depalmitoylation of normal αs (7, 9) and effected transfer of a fraction of αs molecules into cytosol (10–12).
Recent evidence from several laboratories (5, 7, 9, 13), leads us to propose the following biochemical mechanism to explain the receptor-triggered palmitoylation cycle of αs: In this mechanism palmitate plays a dual role, increasing both the hydrophobicity of αs and its affinity for binding βγ. GTP-induced dissociation of βγ increases susceptibility of the α subunit to depalmitoylation; the resulting loss of palmitate decreases intrinsic avidity of αs for the lipid bilayer, promoting its transfer to cytosol. Studies of other G proteins are in keeping with this hypothesis. In all α subunits of the αi family (except αt), covalently attached palmitate and myristate together play this dual role, although one or the other lipid may be selectively responsible for membrane attachment (9, 14–16) or for α/βγ interaction (17–19). Prenylation of the γ polypeptide similarly increases both the hydrophobicity and the affinity of βγ for binding Gα (20, 21). Biochemical and genetic experiments point to a key role in α/βγ interaction for the N termini of Gα subunits (22, 23), to which myristate and/or palmitate are attached. Moreover, crystal structures of G protein trimers show βγ associated with two Gα surfaces, of which one is the N terminal α-helix of Gα (24, 25). The likely proximity of myristate and/or palmitate on the Gα N terminus to the prenyl group at the Gγ C terminus and the proximity of both to the proposed location of the plasma membrane (24, 25) are striking. Together, these findings point to N termini of α subunits as potential physical interaction sites for the proposed cooperation between βγ and lipids, both in attaching Gα to the plasma membrane and also in βγ-mediated prevention of αs from depalmitoylation in intact cells.
To explore the biochemical basis of a mechanism linking membrane attachment, βγ affinity, and the palmitoylation cycle of αs, we compared hydrophobicity and βγ binding affinity of palmitoylated vs. unpalmitoylated recombinant αs and assessed the effect of βγ on depalmitoylation of αs. Our findings provide a clearer picture of mechanism of the palmitoylation cycle and pose questions regarding the potential role(s) of palmitate attached to other subunits.
MATERIALS AND METHODS
Purification of [3H]Palmitoylated αs and β1γ2.
Purification of αs was performed using hexahistidine (H6)-tagged βγ subunit as described (13) with modifications. Sf9 cells (1.5 × 106 cells per ml), maintained in Sf-900II medium at 28°C, were infected with baculoviruses encoding αs, β1, and H6-γ2 (1 plaque-forming unit per cell for each virus) and propagated for 48 h at 28°C. To produce [3H]palmitoylated αs, infected cells were incubated at 107 cells per ml for an additional 2 h in medium containing 5 mM sodium pyruvate and 0.25 mCi/ml [9,10-3H]palmitic acid. Cells were lysed by nitrogen cavitation; membrane fractions were prepared (26). After membrane fractions were extracted with 0.6% (wt/vol) C12E10 (polyoxyethylene 10-lauryl ether, the principal component of Lubrol), supernatant fractions were applied to a column of HiTrap Chelating (Pharmacia; 5 ml bed volume) charged with Ni2+ and equilibrated with buffer A [20 mM Tris·HCl, pH 7.8/100 mM NaCl/1 mM MgCl2/10 μM GDP/1 mM 2-mercaptoethanol/10 mM imidazole/protease inhibitors (27)/0.5% C12E10]. The column was washed with 100 ml of buffer A containing 300 mM NaCl, warmed to room temperature, washed with 15 ml buffer A containing 100 mM NaCl, and then eluted with 30 ml of buffer A containing 1% sodium cholate, 50 mM MgCl2, 30 μM AlCl3, and 10 mM NaF. Finally, for elution of βγ subunits, the column was washed with 20 ml of buffer A containing 300 mM imidazole and 0.7% 3-[(3-cholamindopropyl)dimethylammonio]-1-propanesulfonate. Peak fractions containing αs [AlF4− eluent for αs-wild type (WT) and first 33 ml of high salt wash for αs-C3S] were further chromatographed on a column of Mono Q (Pharmacia; 1-ml bed volume) (28). Fractions were subjected to SDS/PAGE and visualized either by Western blot analysis with anti-αs antibody (10) or by fluorography (7). β1γ2 was purified using H6-αi1, as described (13).
Purification of Cytosolic αs.
To purify αs from cytosol, Sf9 cells were infected with baculovirus encoding αs without β and γ subunit. After cell lysis the supernatant fraction was sequentially chromatographed on columns of HiTrap Q (Pharmacia; 5-ml bed volume × 2) (27), Econo-Pac HTP (Bio-Rad; 5-ml bed volume) with potassium phosphate gradient (29), and Mono Q (1-ml bed volume) (28) in the absence of detergents.
Separation of Palmitoylated and Unpalmitoylated αs by HPLC.
The samples were applied to a C4 reverse-phase column, Synchropak RP-4 (250 × 4.6 mm i.d., Synchrom, Lafayette, IN), equilibrated with 0.1% trifluoroacetic acid and 45% CH3CN at 30°C. The column was eluted at a flow rate of 1.0 ml/min with a linear gradient of CH3CN (45–60%) in 30 min. Fractions (0.5 ml) were assayed for radioactivity and subjected to SDS/PAGE and silver staining.
Triton X-114 Phase Partitioning.
Partitioning of protein samples between aqueous and detergent-rich phases using TX-114 was performed as described (30, 31). Equal volumes of normalized fractions of either water phase (W) or detergent phase (D) were subjected to SDS/PAGE and visualized by Western blot analysis with anti-αs or anti β antibody or by fluorography.
Guanosine 5′-[γ-thio]Triphosphate (GTP[γS]) Binding and GTPase Assays.
GTP[γS] binding or GTPase was quantitated as described (27, 28). Apparent on rates (kapp) of GTP[γS] binding were determined as described (29).
Depalmitoylation Assay.
Depalmitoylation assays were performed as described (32) with modifications. Samples were incubated at 22°C with palmitoyl esterase (a generous gift from Sandra L. Hofmann, University of Texas Southwestern Medical Center, Dallas) in buffer B (50 mM Tris·HCl, pH 7.8/50 mM NaCl/5 mM MgCl2/1 mM EDTA/1 mM 2-mercaptoethanol/10 μM GDP) in the presence of 0.44% octyl glucoside (32). When the rate of depalmitoylation was determined, the reaction was done in the presence of 0.025% C12E10. The [3H]palmitate released into the supernatant was quantitated (32). [3H]Palmitate covalently bound to proteins was analyzed by SDS/PAGE and fluorography.
RESULTS
Purification of Palmitoylated αs.
We infected Sf9 cells with baculoviruses expressing αs-WT or a palmitoylation site mutant (αs-C3S) (8), with or without viruses expressing β1 and a hexahistidine-tagged γ2 subunit (H6-γ2) (13). In the absence of coexpressed βγ, ≈50% of the αs-WT or αs-C3S was found αs-WT in the supernatant fraction (cytosolic αs). Although the cytosolic αs in such extracts was not aggregated, αs in the particulate fraction of cells without coexpressed βγ was probably denatured and aggregated, as indicated by resistance to extraction with 1% sodium cholate or 1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate. In contrast, coexpression of βγ targeted functional αs-WT and αs-C3S (easily solubilized in detergent and capable of binding radioactive GTP[γS]) to membranes. Indeed, both αs-WT and αs-C3S were located predominantly in the particulate fraction when coexpressed with βγ (results not shown). [In situations where recombinant Gα subunits are overexpressed, as in Sf9 cells and in transiently transfected mammalian cells, it is important to determine whether such proteins in particulate fractions are functionally intact, because particulate fractions from such cells often contain nonfunctional G protein subunits that are denatured and aggregated. In our experiments, particulate αs was functionally intact when we coexpressed βγ with αs.] Hereafter termed membrane-derived αs, these proteins were easily solubilized with detergents and served as sources for purified αs-C3S and palmitoylated αs-WT proteins studied in this paper.
Purification of αs from Sf9 cells coexpressing αs, β1, and H6-γ2 provided a strong indication that palmitoylated αs binds more tightly to the βγ subunit than does unpalmitoylated αs (Fig. 1). αsβγ heterotrimers in detergent extracts of membranes were first applied to a Ni2+ column. [3H]palmitoylated αs-WT (from Sf9 cells incubated with [3H]palmitate; see Materials and Methods) was selectively retained on the column and could be eluted with AlF4−. αs-C3S [or unpalmitoylated αs-WT molecules, possibly depalmitoylated during preparation by contaminating palmitoyl esterases (5)] were retained less tightly on the Ni2+ column, apparently because of their lower affinity for βγ (see below); these were eluted in the first washing step (Fig. 1A). Radioactivity incorporated into the protein was shown to be linked to αs-WT by a thioester linkage, because it was quantitatively removed by incubation with either hydroxylamine or palmitoyl esterase (Fig. 1 B and C).
Figure 1.
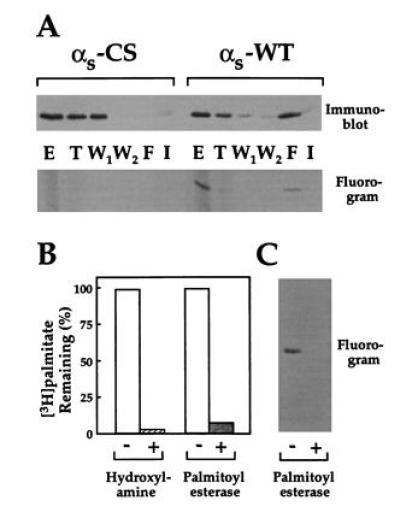
Purification of palmitoylated αs and removal of palmitate. (A) Sf9 cells infected with baculoviruses encoding β1 and H6-γ2 plus either αs-WT or αs-C3S were incubated with [3H]palmitate and lysed. Detergent extracts (in the nonionic detergent C12E10) from particulate fractions were chromatographed on a Ni2+ column as described. Aliquots of the initial detergent extract (E), flow through (T), washes with high and low salt (W1, first 33 ml, and W2), AlF4− eluent (F), and imidazole eluent (I) were subjected to SDS/PAGE and immunoblotted with anti-αs antibody or visualized by fluorography. (B and C) Purified [3H]palmitoylated αs-WT (100 nM, 210 cpm/pmol) was incubated at 25°C for 7 h with 1 M Tris·HCl (pH 7.0) or 1 M hydroxylamine (pH 7.0) or was treated with palmitoyl esterase (0.7 μg/ml) for 50 min. Released radioactivity was determined, and the remaining protein-bound radioactivity was calculated (B) or visualized by SDS/PAGE followed by fluorography (C).
αs-WT from the AlF4− elution and αs-C3S in the first wash fraction were further purified by passage over a Mono-Q column, as described in Materials and Methods.
Separation of Palmitoylated vs. Unpalmitoylated αs.
Palmitoylated and unpalmitoylated αs migrated separately on a reverse phase C4 HPLC column (Fig. 2), in a fashion consistent with their presumed difference in hydrophobicity. αs-WT eluted from the column in two reproducible peaks (Fig. 2A, peaks I and II). [3H]Palmitate-labeled αs-WT eluted in peak II (at a higher concentration of CH3CN), while unpalmitoylated αs-WT (lacking [3H]palmitate), αs-WT derived from Sf9 cytosol, and αs-C3S all migrated in peak I (Fig. 2 A and B). Relative sizes of peaks I and II from αs-WT preparations indicated that ≈50% of αs-WT derived from Sf9 membranes is linked to palmitate. (Note: SDS/PAGE of peaks I and II revealed, in silver-stained gels, one protein at the size expected for αs.)
Figure 2.
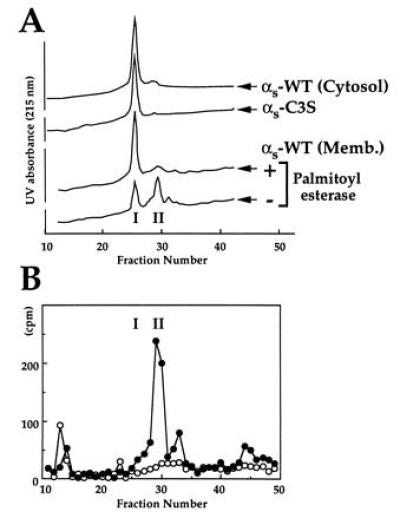
Separation of palmitoylated vs. unpalmitoylated αs by reverse-phase chromatography. (A) Ten picomoles of cytosolic αs-WT, αs-C3S, and membrane-derived αs-WT (purified [3H]palmitoylated αs) were chromatographed on a C4 HPLC reverse-phase column. (B) Radioactivity in column fractions after chromatography (as in A) of purified [3H]palmitoylated αs-WT incubated with (○, 10 pmol of total protein) or without (•, 5 pmol of total protein) palmitoyl esterase.
Incubation of membrane-derived αs-WT with palmitoyl esterase caused disappearance of the [3H]palmitoylated protein in peak II (Fig. 2B, closed vs. open symbols) and a corresponding increase in (unpalmitoylated) protein migrating in peak I (Fig. 2A).
Palmitoylation Increases Hydrophobicity of αs and Its Affinity for βγ.
The idea that palmitoylation enhances hydrophobicity of αs is in accord with localization of αs-C3S in cytosol of intact cells and the migration of αs-WT from membrane to cytosol that accompanies activation-induced depalmitoylation (7, 8, 12). Selective partitioning of membrane-derived αs-WT in TX-114 confirms this suggestion (Fig. 3). Most (77%) of the [3H]palmitate linked to αs-WT partitioned into the detergent phase, while αs-C3S, cytosol-derived αs-WT, and palmitoyl esterase-treated αs-WT partitioned almost exclusively into the water phase. In agreement with the 50% stoichiometry of palmitoylation in membrane-derived αs-WT (Fig. 2), total αs-WT protein partitioned 60% into water and 40% into detergent, as assessed by densitometry of immunoblotted protein (Fig. 3). In keeping with its hydrophobicity, prenylated βγ partitioned exclusively into detergent (Fig. 3), while a water-soluble control protein, bovine serum albumin, partitioned exclusively into water (not shown).
Figure 3.
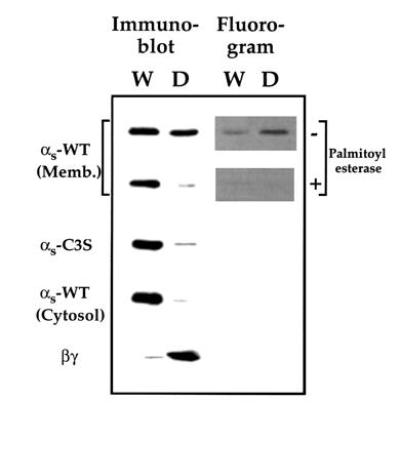
Triton X-114 phase partitioning to assess the hydrophobicity of palmitoylated and unpalmitoylated αs. Membrane-derived palmitoylated αs-WT incubated without or with palmitoyl esterase, αs-C3S, cytosol-derived αs-WT, or βγ subunit (each at 50 nM) were subjected to TX-114 phase partitioning as described. Equal volumes of normalized fractions of either water phase (W) or detergent phase (D) were subjected to SDS/PAGE and visualized either by immunoblotting with anti-αs or anti-β antibody or by fluorography.
Because our purification procedure suggested that palmitoylated αs binds βγ more tightly than does unpalmitoylated αs (Fig. 1), we directly assessed the effect of palmitoylation on the interaction of αs with βγ. βγ slows binding of GTP to αs, apparently by inhibiting dissociation of bound GDP (29). We therefore measured the apparent rate of association of GTP[γ35S] with αs in the presence of different concentrations of βγ (Fig. 4). As expected, the IC50 of this inhibitory effect was consistently ≈5-fold higher with unpalmitoylated αs (αs-C3S, cytosol-derived αs-WT, or αs-WT treated with palmitoyl esterase) than with membrane-derived αs-WT, in which half the αs molecules are palmitoylated. Thus palmitoylation increases the apparent affinity of αs for βγ. In the absence of βγ, GTP[γS] bound to αs-WT and αs-C3S at similar rates (0.19 vs. 0.22 min−1). Parallel experiments (not shown) testing the IC50 of βγ for inhibition of steady state GTP hydrolysis by αs revealed quantitatively similar differences between palmitoylated and unpalmitoylated αs.
Figure 4.
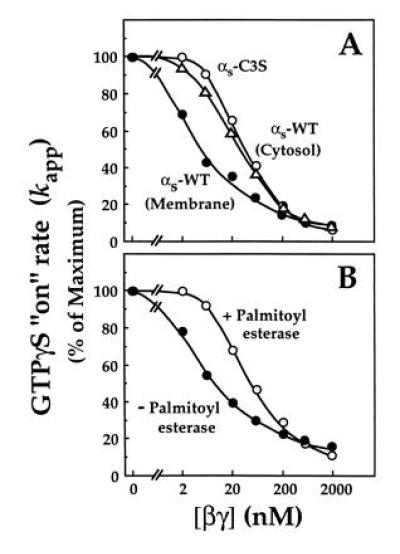
Effect of βγ subunit on rate of association of GTP[γS] with palmitoylated and unpalmitoylated αs. Recombinant βγ at the indicated concentrations was incubated with (A) membrane-derived (palmitoylated) αs-WT (•), αs-C3S (○), or cytosol-derived αs-WT (▵) or (B) with membrane-derived (palmitoylated) αs previously incubated in the absence (•) or presence (○) of palmitoyl esterase; in each case the concentration of αs was 10 nM. Each αs preparation was incubated at 22°C with 1 μM GTP[γ35S] (105 cpm/pmol) and apparent on-rates of GTP[γS] binding (kapp) were calculated as described. Boiled βγ (2 μM) did not produce any inhibition (not shown).
βγ Slows Depalmitoylation of αs.
Activation of αs accelerates its depalmitoylation in intact cells (7). Hypothetically, increased susceptibility of activated αs to action of cellular palmitoyl esterases could result from GTP-induced conformational change; alternatively, if βγ protects against these esterases, accelerated depalmitoylation could be caused by activation-induced dissociation of αs from βγ. The results shown in Fig. 5 indicate that the latter hypothesis is correct. βγ substantially slowed depalmitoylation of αs·GDP by a purified recombinant palmitoyl esterase (Fig. 5A). βγ had no effect on αs depalmitoylation in the presence of GTP[γS] (Fig. 5B), presumably because binding of the GTP analog caused αs to dissociate from βγ. In the absence of βγ, however, αs·GTP[γS] and αs·GDP were depalmitoylated at the same rate (Fig. 5C).
Figure 5.
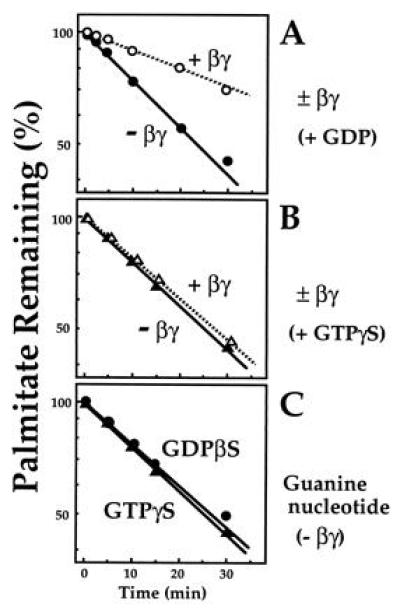
Effect of βγ and guanine nucleotides on depalmitoylation of αs by palmitoyl esterase. [3H]Palmitoylated membrane-derived αs-WT (100 nM) was incubated at 22°C with palmitoyl esterase (0.14 μg/ml) in buffer B in the presence of 0.025% C12E10. At the times indicated, reactions were terminated, released [3H]palmitate was quantitated, and remaining αs-bound palmitate was calculated. (A) GDP-bound [3H]palmitoylated αs-WT was incubated in the absence of MgCl2 and in the presence of recombinant βγ (○) or boiled βγ (•) (500 nM each). (B) [3H]Palmitoylated αs-WT was incubated with palmitoyl esterase with recombinant βγ (○) or boiled βγ (•) (500 nM each). Before the assay, the [3H]palmitoylated protein was incubated with 15 μM GTP[γS] in the presence of 15 mM MgCl2 at 22°C for 50 min. (C) [3H]Palmitoylated αs-WT was incubated with palmitoyl esterase in the absence of βγ. Before the assay, the [3H]palmitoylated protein was incubated either with 15 μM GDP[βS] (•) or with 15 μM GTP[γS] (▴) in the presence of 15 mM MgCl2 at 22°C for 50 min.
DISCUSSION
Our observations demonstrate reciprocal relations between βγ binding to αs and palmitoylation of this Gα subunit. As compared with the unpalmitoylated protein, palmitoylated αs associates more tightly with βγ during purification (Fig. 1) and has a higher apparent affinity for βγ in vitro, as indicated by the decreased IC50 for βγ inhibition of GTP[γS] binding (Fig. 4). Conversely, βγ protects αs from depalmitoylation by palmitoyl esterase (Fig. 5). In addition, our observations are the first to confirm directly the inference that the presence or absence of attached palmitate determines the location of αs in membranes vs. cytosol (Figs. 2, 3, 4). Here we discuss biochemical mechanisms underlying this reciprocal interaction, its implications for the activation-regulated palmitoylation cycle of αs (Fig. 6) and membrane localization of the Gs heterotrimer, and comparisons of αs to other Gα subunits.
Figure 6.
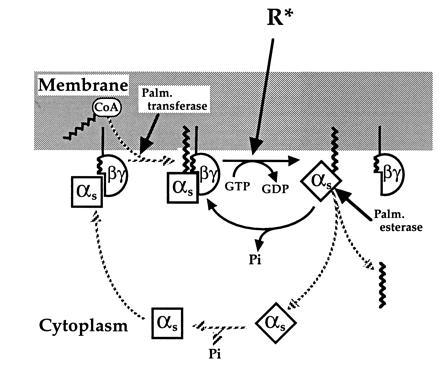
Model of the αs palmitoylation cycle. In the resting state, αs is held at the membrane by the hydrophobic interaction of its attached palmitate with the bilayer and by association with βγ, which is itself attached to the membrane. In addition, palmitoylation enhances affinity of αs for βγ and βγ reciprocally promotes the palmitoylated state by protecting αs from attack of palmitoyl esterase and by enhancing palmitoylation catalyzed by palmitoyl transferase. Receptor-triggered activation of αs induces its translocation to cytoplasm by causing subunit dissociation and rapid depalmitoylation. After converting bound GTP to GDP, cytosolic αs returns to the membrane by binding βγ, followed by repalmitoylation by the transferase. In comparison to our previous model (3), the proposed cycle stresses reciprocal regulation by palmitate and βγ and specifies the order of steps required for reattachment of cytosolic αs to the membrane.
Biochemical Mechanism of Reciprocal Relations Between βγ and Palmitate.
Three-dimensional structures of transducin (25) and Giα (24) show that the N terminal α helix of Gα provides an important binding interface with βγ. This surface is very close to the likely sites of Gα lipid modifications at or near the protein’s extreme N terminus [although these modifications are absent in available crystals (24, 25)]. Thus the most obvious explanation for reciprocity between βγ and palmitate on αs is that the hydrophobic lipid attached to αs directly contributes binding energy to the αs/βγ interaction, and that, in turn, βγ shields the lipid from attack by esterase.
An additional possibility, which does not exclude the first, is that the increased hydrophobicity of palmitoylated αs (Fig. 3) acts indirectly: Palmitate would enhance avidity of αs for membrane bilayers or detergent micelles, potentiating the αs/βγ interaction by increasing αs concentration in physical proximity to membrane-bound or detergent-solubilized βγ. βγ would reciprocally enhance association of palmitoylated αs with membrane or detergent, and proximity of the αs N terminus to the lipid bilayer could decrease its accessibility to action of the esterase. To our knowledge such indirect effects of lipid attachment have not been ruled out for any Gα/βγ interaction; all reported studies of such interactions (summarized in table 1 of ref. 3) have been carried out with proteins attached to cell membranes or associated with detergent micelles.
Finally, reciprocity could result from palmitate- and βγ-dependent changes in the conformation of αs. Indeed, association with βγ stabilizes the N-terminal α-helix of Gα subunits in crystals (24, 25). It is also possible that attached palmitate similarly stabilizes a Gα conformation with higher affinity for βγ—an untested possibility, in that none of the crystals so far reported contains palmitoylated Gα.
Our data are consistent with all three mechanisms. Although to us the first two appear more likely, all three could combine to produce the reciprocal relations of βγ and palmitate with respect to αs.
The Palmitoylation Cycle and Membrane Localization of αs.
Our in vitro findings, combined with evidence from in vivo experiments, suggest a biochemical basis for the palmitoylation cycle of αs (Fig. 6): In the resting state, palmitoylated αs is held at the plasma membrane by a combination of increased hydrophobicity due to the lipid modification and enhanced affinity for binding membrane-bound βγ. The GTP-triggered conformational change in αs that accompanies Gs activation induces subunit dissociation, releasing αs from the protective embrace of βγ and making αs accessible to palmitoyl esterases. Resulting depalmitoylation decreases avidity of αs for the membrane bilayer, allowing translocation of the relatively hydrophilic αs molecule from membrane to cytosol. After GTP hydrolysis αs·GDP returns to the membrane by binding βγ. Indeed, βγ reportedly (33, 34) enhances susceptibility of Gα proteins to the forward reaction, palmitoylation. Thus localization of αs in membranes depends upon reciprocal potentiation of its membrane avidity by βγ and attached palmitate, and loss of either part of this dual regulation promotes translocation of the protein from membrane to cytosol—and vice versa.
In a possible addition to this scenario, attached palmitate could play a key role in the well-established (35) selective association of αs with the plasma membrane in preference to membranes of intracellular organelles. In keeping with this notion, palmitoyl transferase activity is much more abundant in the plasma membrane fraction of liver homogenates (33). Thus even if βγ were not selectively located in the plasma membrane (relative to other cellular membranes), palmitoylation occurring exclusively or predominantly at that site would retain αs close to receptors and effectors at the cell surface by stabilizing its association with βγ and the lipid bilayer.
Palmitate on Other α Subunits.
Although αq, like αs, is palmitoylated but not myristoylated, the palmitate on αs appears to play a more important role in determining hydrophobicity, α/βγ interaction, and membrane avidity. In the case of αq, other N-terminal residues contribute significantly to hydrophobicity, over and above the effect of palmitates attached to cysteines at positions 9 and 10 (31). Even in its unpalmitoylated state αq is sufficiently hydrophobic to partition almost equally into the detergent and water phases of TX-114; enzymatic removal of the of N-terminal 34 residues of αq, however, cause it to partition completely into the water phase (31). Although the C9,10S mutation impaired its activation of phospholipase C (8, 31), treatment of palmitoylated αq with palmitoyl esterase failed to alter interactions of αq with receptor, βγ, and phospholipase C (31). For this reason, the effects of the mutation were attributed to replacement of cysteines by serines, rather than to absence of palmitate (31). This negative result may be partly explained by the relatively hydrophobic nature of αq even in the absence of palmitoylation; in addition, an apparently low stoichiometry of αq palmitoylation (20–40%, ref. 31) may have obscured a real functional effect of palmitate.
In the αi family Gα subunits are irreversibly attached to myristate at a glycine residue just upstream of the cysteine to which palmitate is linked. Although both lipid modifications are likely to contribute to βγ affinity and avidity for membranes, their relative and/or specific roles are just beginning to be explored (3, 5, 16). It should be noted in this connection that hydrophobicity contributed by myristate is only marginally sufficient for assured association with cellular membranes, in contrast to palmitate, which is longer and more hydrophobic (36). The dual mechanisms of membrane attachment by structures near the N termini of αq and αi resemble those mediated by structures at or near the C termini of Ras proteins: a polybasic domain (Ki-ras) or palmitoylation (Ha- and N-ras) is required in addition to isoprenylation for localization to the plasma membrane (37).
We are just beginning to explore how different lipid modifications and peptide sequences regulate membrane avidity and localization of Gα proteins. Success in this task will eventually help to elucidate mechanisms responsible for localizing a much larger array of proteins at the cytoplasmic surface of cell membranes.
Acknowledgments
We thank Michael Degtyarev, Simone Fishburn and John Silvius for useful advice; Tohru Kozasa and Alfred G. Gilman for sharing methods for purifying recombinant α subunits and for providing baculoviruses encoding β1, γ2, H6-γ2, and H6-αi1; and Sandra L. Hofmann for purified palmitoyl esterase.
Footnotes
Abbreviations: WT, wild type; GTP[γS], guanosine 5′[γ-thio]triphosphate.
References
- 1.Conklin B R, Bourne H R. Cell. 1993;73:631–641. doi: 10.1016/0092-8674(93)90245-l. [DOI] [PubMed] [Google Scholar]
- 2.Neer E J. Cell. 1995;80:249–257. doi: 10.1016/0092-8674(95)90407-7. [DOI] [PubMed] [Google Scholar]
- 3.Wedegaertner P B, Wilson P T, Bourne H R. J Biol Chem. 1995;270:503–506. doi: 10.1074/jbc.270.2.503. [DOI] [PubMed] [Google Scholar]
- 4.Casey P J. Curr Opin Cell Biol. 1994;6:219–225. doi: 10.1016/0955-0674(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 5.Ross E M. Curr Biol. 1995;5:107–109. doi: 10.1016/s0960-9822(95)00026-1. [DOI] [PubMed] [Google Scholar]
- 6.Linder M E, Middleton P, Hepler J R, Taussig R, Gilman A G, Mumby S M. Proc Natl Acad Sci USA. 1993;90:3675–3679. doi: 10.1073/pnas.90.8.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wedegaertner P B, Bourne H R. Cell. 1994;77:1063–1070. doi: 10.1016/0092-8674(94)90445-6. [DOI] [PubMed] [Google Scholar]
- 8.Wedegaertner P B, Chu D H, Wilson P T, Levis M J, Bourne H R. J Biol Chem. 1993;268:25001–25008. [PubMed] [Google Scholar]
- 9.Mumby S M, Kleuss C, Gilman A G. Proc Natl Acad Sci USA. 1994;91:2800–2804. doi: 10.1073/pnas.91.7.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levis M J, Bourne H R. J Cell Biol. 1992;119:1297–1307. doi: 10.1083/jcb.119.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ransnäs L A, Svoboda P, Jasper J R, Insel P A. Proc Natl Acad Sci USA. 1989;86:7900–7903. doi: 10.1073/pnas.86.20.7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wedegaertner P B, Bourne H R, von Zastrow M. Mol Biol Cell. 1996;7:1225–1233. doi: 10.1091/mbc.7.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kozasa T, Gilman A G. J Biol Chem. 1995;270:1734–1741. doi: 10.1074/jbc.270.4.1734. [DOI] [PubMed] [Google Scholar]
- 14.Hallak H, Brass L F, Manning D R. J Biol Chem. 1994;269:4571–4576. [PubMed] [Google Scholar]
- 15.Degtyarev M Y, Spiegel A M, Jones T L Z. J Biol Chem. 1994;269:30898–30903. [PubMed] [Google Scholar]
- 16.Wilson P T, Bourne H R. J Biol Chem. 1995;270:9667–9675. doi: 10.1074/jbc.270.16.9667. [DOI] [PubMed] [Google Scholar]
- 17.Linder M E, Pang I-H, Duronio R J, Gordon J I, Sternweis P C, Gilman A G. J Biol Chem. 1991;266:4654–4659. [PubMed] [Google Scholar]
- 18.Jones T L, Simonds W F, Merendino J J, Brann M R, Spiegel A M. Proc Natl Acad Sci USA. 1990;87:568–572. doi: 10.1073/pnas.87.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kokame K, Fukada Y, Yoshizawa T, Takao T, Shimonishi Y. Nature (London) 1992;359:749–752. doi: 10.1038/359749a0. [DOI] [PubMed] [Google Scholar]
- 20.Muntz K H, Sternweis P C, Gilman A G, Mumby S M. Mol Biol Cell. 1992;3:49–61. doi: 10.1091/mbc.3.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iñiguez-Lluhi J A, Simon M I, Robishaw J D, Gilman A G. J Biol Chem. 1992;267:23409–23417. [PubMed] [Google Scholar]
- 22.Fung B K-K, Nash C R. J Biol Chem. 1983;258:10503–10510. [PubMed] [Google Scholar]
- 23.Graf R, Mattera R, Codina J, Estes M K, Birnbaumer L. J Biol Chem. 1992;267:24307–24314. [PubMed] [Google Scholar]
- 24.Wall M A, Coleman D E, Lee E, Iñiguez-Lluhi J A, Posner B A, Gilman A G, Sprang S R. Cell. 1995;83:1047–58. doi: 10.1016/0092-8674(95)90220-1. [DOI] [PubMed] [Google Scholar]
- 25.Lambright D G, Sondek J, Bohm A, Skiba N P, Hamm H E, Sigler P B. Nature (London) 1996;379:311–9. doi: 10.1038/379311a0. [DOI] [PubMed] [Google Scholar]
- 26.Iiri T, Homma Y, Ohoka Y, Robishaw J D, Katada T, Bourne H R. J Biol Chem. 1995;270:5901–5908. doi: 10.1074/jbc.270.11.5901. [DOI] [PubMed] [Google Scholar]
- 27.Iiri T, Herzmark P, Nakamoto J M, Van Dop C, Bourne H R. Nature (London) 1994;371:164–168. doi: 10.1038/371164a0. [DOI] [PubMed] [Google Scholar]
- 28.Iiri T, Ohoka Y, Ui M, Katada T. J Biol Chem. 1992;267:1020–1026. [PubMed] [Google Scholar]
- 29.Graziano M P, Freissmuth M, Gilman A G. J Biol Chem. 1989;264:409–418. [PubMed] [Google Scholar]
- 30.Bourdier C. J Biol Chem. 1981;256:1604–1607. [PubMed] [Google Scholar]
- 31.Hepler J R, Biddlecome G H, Kleuss C, Camp L A, Hofmann S L, Ross E M, Gilman A G. J Biol Chem. 1996;271:496–504. doi: 10.1074/jbc.271.1.496. [DOI] [PubMed] [Google Scholar]
- 32.Camp L A, Verkruyse L A, Afendis S J, Slaughter C A, Hofmann S L. J Biol Chem. 1994;269:23212–23219. [PubMed] [Google Scholar]
- 33.Dunphy J T, Greentree W K, Manahan C L, Linder M E. J Biol Chem. 1996;271:7154–7159. doi: 10.1074/jbc.271.12.7154. [DOI] [PubMed] [Google Scholar]
- 34.Duncan J A, Gilman A G. J Biol Chem. 1996;271:23594–23600. doi: 10.1074/jbc.271.38.23594. [DOI] [PubMed] [Google Scholar]
- 35.Wilson B S, Komuro M, Farquhar M G. Endocrinology. 1994;134:233–243. doi: 10.1210/endo.134.1.8275939. [DOI] [PubMed] [Google Scholar]
- 36.Peitzsch R M, McLaughlin S. Biochemistry. 1993;32:10436–10443. doi: 10.1021/bi00090a020. [DOI] [PubMed] [Google Scholar]
- 37.Hancock J F, Paterson H, Marshall C J. Cell. 1990;63:133–139. doi: 10.1016/0092-8674(90)90294-o. [DOI] [PubMed] [Google Scholar]


