Abstract
Expression of G protein-regulated phospholipase C (PLC) β4 in the retina, lateral geniculate nucleus, and superior colliculus implies that PLC β4 may play a role in the mammalian visual process. A mouse line that lacks PLC β4 was generated and the physiological significance of PLC β4 in murine visual function was investigated. Behavioral tests using a shuttle box demonstrated that the mice lacking PLC β4 were impaired in their visual processing abilities, whereas they showed no deficit in their auditory abilities. In addition, the PLC β4-null mice showed 4-fold reduction in the maximal amplitude of the rod a- and b-wave components of their electroretinograms relative to their littermate controls. However, recording from single rod photoreceptors did not reveal any significant differences between the PLC β4-null and wild-type littermates, nor were there any apparent differences in retinas examined with light microscopy. While the behavioral and electroretinographic results indicate that PLC β4 plays a significant role in mammalian visual signal processing, isolated rod recording shows little or no apparent deficit, suggesting that the effect of PLC β4 deficiency on the rod signaling pathway occurs at some stage after the initial phototransduction cascade and may require cell–cell interactions between rods and other retinal cells.
Keywords: animal behavior, vision, transgenic mice, retinal function, visual response
Many hormones, neurotransmitters, and other biologically active molecules transduce signals by activating phospholipase C (PLC) through heterotrimeric GTP-binding proteins (G protein) (1, 2). PLC hydrolyzes phosphatidylinositol 4,5-bisphosphate to generate two important second messengers: inositol triphosphate and diacylglycerol (3). The Gq class of G proteins, including Gαq, Gα11, Gα14, Gα15, and Gα16, activate the β class of PLC enzymes (4–12). Molecular cloning has revealed four isoforms, PLC β1-4. The β4 isoform is found in the retina (13, 14) and in the brain (13, 15). PLC β4 has been localized to the photoreceptors, bipolar cells, horizontal cells, and ganglion cells of the bovine retina by immunohistochemical staining (14). Intriguingly, PLC β4 shares closer homology than PLC β1-3 with the NorpA protein, which mediates the phototransduction cascade in Drosophila photoreceptors (16). However, the role of PLC β4 in mammalian visual processes is not yet clear; hydrolysis of cGMP by G protein-regulated phosphodiesterase is established as the signal transduction pathway that mediates visual signal processing in photoreceptors (17). We have examined the effects of disrupting the PLC β4 gene on vision; changes in electroretinograms (ERGs) and in behavior suggest that PLC β4 plays a role in a step in visual processing that occurs after the initial photocascade in the rod outer segment and might feedback to effect the retinal response in situ.
MATERIALS AND METHODS
Shuttle Box Test.
The shuttle box test was performed basically as described (18). The mice were placed in a shuttle box, which is divided into two compartments. An initial conditioned stimulus (CS, sound or light flash) was applied followed after 10 sec by an unconditioned stimulus (US, electric foot-shock, 0.5 mA). One hundred trials were performed each day. The mice with auditory and visual abilities can learn to associate sound and light, respectively, with the unconditional stimulus, and cross into the other compartment within 10 sec of conditional stimulus onset without receiving a foot-shock.
ERG Recording.
For recording methods and intensity calibrations see Lyubarsky and Pugh (19). The mice were anesthetized and their pupils were dilated. Light flashes were calculated to produce, from dimmest to most intense, the numbers of photoisomerizations as shown in the figures. Average responses of wild-type and PLC β4-null rods to 10 msec flashes of increasing strength. Stimulus timing is shown by flash monitor below records. Responses were normalized by the saturating response amplitudes, rmax (Table 1). Flash strengths were: wild-type, 8.34, 53.7, 99.4, 361, and 1210 photons/μm2; PLC β4-null, 13.3, 44.2, 98.1, 161, 297, and 3640 photons/μm2 at 500 nm.
Table 1.
Properties of electrical responses of PLC β4-null mice and wild-type controls
| Genotype | amax, μV | bmax, μV | rmax, pA | i0, photons/μm2 |
|---|---|---|---|---|
| +/+ | 320 ± 155 (4) | 790 ± 380 (4) | 8.9 ± 3.0 (11) | 60 ± 10 (10) |
| −/− | 77 ± 26 (5) | 168 ± 60 (5) | 8.9 ± 2.8 (96) | 50 ± 27 (77) |
amax and bmax denote the maximum amplitudes of the a-wave and the scotopic (rod-mediated) b-wave of the ERG, respectively. rmax is the amplitude of the maximal response determined by suction electrode recording from single rods (20, 21). i0 is the half-saturating flash strength, an inverse measure of rod flash sensitivity, obtained by fitting the relation between response amplitude and flash strength with the saturating exponential (r/rmax = 1 − exp(iln2/i0), where r is the response amplitude and i is the flash intensity in photons μm−2 at 500 nm. The number of photoisomerizations that gave a half-saturating response can be found by multiplying i0 by the rod collecting areas, ≈0.25 μm2. Error are SDs. Numbers in brackets give total animals for ERGs and total rods for photocurrents. The rods for photocurrents were from 3 PLC β4-null mice and 35 wild-type mice.
Retinal Morphology.
Wild-type and PLC β4-null mice used for ERG recordings were deeply anesthetized, and their eyes were enucleated. The eye was incised at the equator, and fixed in a mixture of 2% paraformaldehyde and 2% glutaraldehyde in phosphate buffer. A central square (about 1.4 × 1.4 mm2 around the optic disk) was cut out, dehydrated, embedded in Epon, and sectioned radially. Sections (0.5–0.7 μm) were collected every 100 μm at the periphery and every 25 μm near the center; they were stained with toluidine blue, and retinal thickness at different locations was measured.
RESULTS
To understand the significance of PLC β4-linked pathways in mammalian visual processes, we generated a mouse line that lacks PLC β4 using the standard protocol (H.J., M.I.S., and D.W., unpublished work). The exon of the PLC β4 gene, which encodes part of the X box was disrupted by the insertion of a neomycin-resistant gene-expression unit. The X box, together with the Y box, comprises the catalytic domain of the PLC activity (22). Thus, the disrupted gene encodes only an N-terminal fragment of PLC β4; hence, the gene product should not be active. The retinal homogenates from a PLC β4-null mouse and a wild-type mouse were analyzed by Western blotting with a PLC β4-specific antibody. No PLC β4 protein was detected in the retinal homogenate derived from the PLC β4-null mouse (Fig. 1A). We also examined the expression of PLC β4 in mouse brains by immunohistochemical staining. PLC β4 is expressed at high levels in the lateral geniculate nucleus and superior colliculus, as well as the mammillary nucleus (Fig. 1B). PLC β4 proteins were not detected in the PLC β4-null mice (Fig. 1B). These results indicate that PLC β4 is expressed in the visual pathway.
Figure 1.
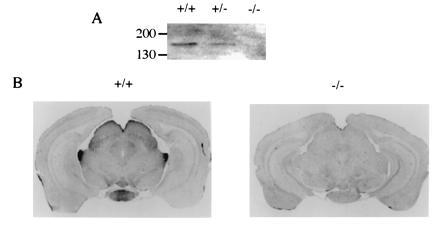
Expression of PLC β4 in visual pathway. (A) Western blot analysis of retinal extracts. Retinal homogenates from a wild-type mouse (+/+) and from mice homozygous (−/−) and heterozygous for disrupted PLC β4 gene were analyzed by Western blotting with the PLC b4-specific antibody. (B) Immunohistochemical staining of brain sections. The wild-type and PLC β4-null mice were perfused with fixative and their brain sections were prepared and stained with a PLC β4-specific antibody using a Vectastain kit (Vector Laboratories).
To investigate the impact of the PLC β4 deficiency on processing of visual data, we performed a shuttle box avoidance learning test in conjunction with either light or sound stimulation. The mice were first tested with sound as the CS and foot-shock as the US. They were then tested with light as the CS. As shown in Fig. 2, both the mutant mice and wild-type mice can learn to avoid foot-shock and the percentage of successful avoidance improved in subsequent trial days with sound as the CS, suggesting that these mice retained their auditory capabilities and memories. However, the PLC β4-null mice were incapable of learning to avoid foot-shock when light was used as the CS, whereas the wild-type mice still could (Fig. 2). The mutant mice were then tested again with sound as the CS; they were still able to respond to sound and avoid foot-shock with similar success rates as when they were tested before light was used as CS. These results indicate that the PLC β4-deficient mice were unable to associate light with foot-shock, although they were able to associate sound with the US. Therefore, it is reasonable to conclude that the PLC β4-linked pathways are essential for visual signal processing.
Figure 2.
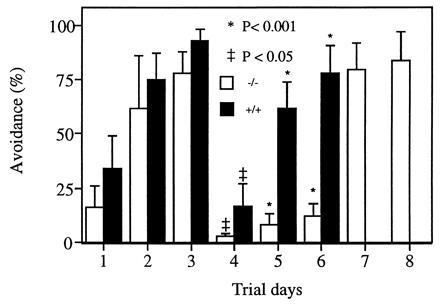
Shuttle box avoidance test. The avoidance tests were carried out with sound as the CS in the first 3 days. Then light (60 W) was used as the CS for the following 3 days. Finally, the mutant mice were tested for 2 more days with sound as the CS. Eight wild-type mice and five PLC β4-null mice were tested. Data were expressed as means ± SEM and were compared between groups by a non-parametric analysis of variance for unrelated samples (Mann–Whitney U-test).
The expression of PLC β4 in the retina led us to investigate the retinal function of the PLC β4-null mice with electroretinography. Analysis of the ERG indicates that both the a-wave and b-wave of five homozygotes (about 3 months old) were reduced compared with those of their wild-type littermates (Fig. 3A, Table 1). The ERG a-wave component in mouse is a field potential generated by the photocurrents in the rods (19), whereas the b-waves characterized here originated largely from the bipolar cells (18, 23, 24). Thus, the abnormalities in ERGs of these PLC β4-null mice suggest that PLC β4 plays a significant role in the distal rod-mediated signaling in the retina. The possibility that the observed deficits in the ERGs of the mutants were caused by the loss of rod photoreceptors was excluded by light microscopic examination of retinas immediately after the ERG recording; the mutant retinas showed no apparent histological abnormalities compared with the ones from their wild-type littermates (Fig. 4). In particular, there were no obvious changes in either rod outer segment length or in the numbers of nuclei in the outer nuclear layer. Thus, the attenuation of ERG amplitudes was not caused by the direct loss of rod photoreceptors or by loss of the outer segment membrane that carries the cGMP-activated current.
Figure 3.
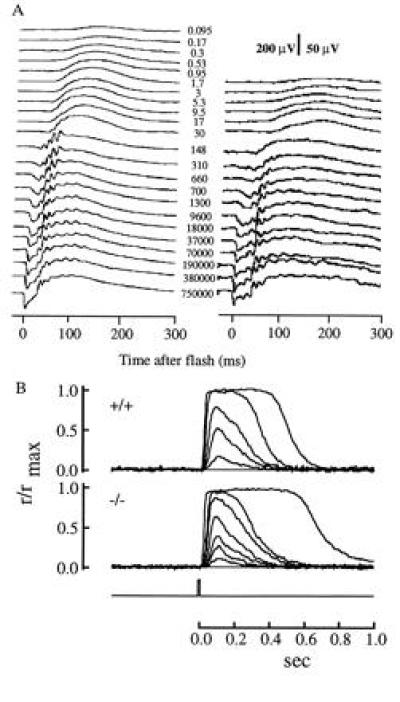
ERG recordings and morphology of PLC β4-null mice. (A) Families of full-field corneal ERGs from a control (Left) and a PLC β4-null mouse (Right). Note the 4-fold difference in amplitude scales for the two sets of data. The numbers near the traces are estimates of the photoisomerizations/rod produced by the stimulating flashes. Each plotted trace is the average of 2–5 individual records. (B) Average responses of wild-type and PLC β4-null rods to 10-msec flashes of increasing strength. Stimulus timing shown by flash monitor below records. Responses were normalized by the saturating response amplitudes, rmax (Table 1). Flash strengths were: wild-type, 8.34, 53.7, 99.4, 361, and 1210 photons/μm2; PLC β4-null, 13.3, 44.2, 98.1, 161, 297, and 3640 photons/μm2 at 500 nm.
Figure 4.
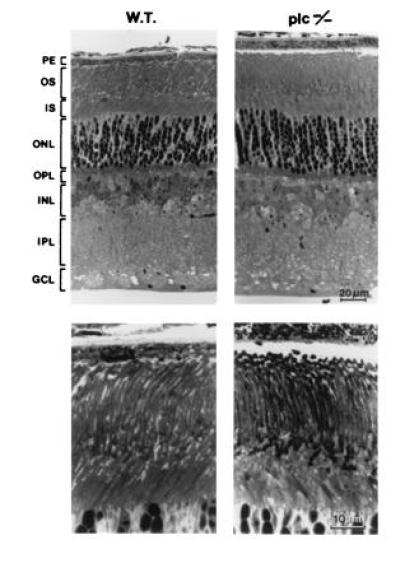
Retinal morphology. (Upper) Radial sections in lower magnification. (Lower) Photoreceptors at higher magnification. For both wild-type and mutant there was little variation across retinal eccentricities; average thickness for wild type was 188 ± 8 and for the mutant 208 ± 3. In addition, retinal morphology in the PLC β4-null mutant (Right) appeared normal compared with that in the wild-type mouse. PE, pigment epithelium; OS, outer segment; IS, inner segment; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer.
Another hypothesis that might account for the diminished ERGs is that PLC β4 plays a role in the rod phototransduction cascade. To examine this hypothesis, the photocurrents of single rods from wild-type and mutant mice were recorded with the suction electrode technique (Fig. 3B, Table 1). No significant differences were found between the maximum photocurrents of populations comprising 11 rods from the mutants and 96 rods from wild-type controls, nor were there any differences between the populations in sensitivity (Table 1). These results indicate that PLC β4 does not appear to have a direct effect on the phototransduction cascade that regulates the cation channels of the rods. It thus appears that the diminution of rod visual function manifest in the ERG a- and b-waves of PLC β4 mutants requires the presence of various other cellular structures and is not the result of a deficiency in the isolated rods.
DISCUSSION
The results reported here show that PLC β4, like its homolog in Drosophila, plays a role in visual signaling in mammals. The shuttlebox experiments demonstrate that PLC β4-null mice have lost important visual processing capabilities, and that neither sensory processing in general, nor learning per se, are the cause of their deficiency. The ERG data reveal that rod signaling pathways in the PLC-β4 mutants are compromised in vivo. Previous work shows that mice lacking expression of the rod-to-rod-bipolar metabotropic coupling receptor mGluR6 can still learn a visual shuttlebox task as well as littermate controls (18). Thus, complete loss of the rod-bipolar pathway (and of the scotopic b-wave, which originates in rod-bipolars) is not sufficient to eliminate visual-mediated learning; mGluR6-null mice likely use signals from cones or signals from rods traveling an alternative pathway than that through the rod-bipolars in such learning. In contrast, in PLC β4-null mice, all visual processing appears affected. The expression of PLC β4 at high levels in lateral geniculate nucleus and superior colliculus also raises a possibility that PLC β4 deficiency may interrupt the signal transmission from retina to higher centers in the nervous system.
While there can be little doubt that PLC β4 is involved in retinal function, especially of the rod-to-rod-bipolar signaling pathway, the lack of any significant changes in the response properties of isolated rods and the presence of residual rod-mediated a- and b-waves in the PLC β4-null mice suggests that PLC β4 plays only a modulatory role in the rod and rod-bipolar signaling cascades, rather than acting as a direct intermediary as it does in Drosophila phototransduction. If PLC β4 directly mediated signal transduction in either the rod or rod-bipolar, we would have expected complete loss of the a- and/or b-wave, respectively, and in the former case, loss of the responsiveness of isolated rods. Activation of PLC β4 is generally found to be via a G protein of the Gq class, whereas the rod cascade is mediated by Gt (transducin). Our observations thus lead to the hypothesis that PLC β4 is involved in rod and possibly rod-bipolar signal transduction indirectly, by modulating one or more regulatory steps involving other retinal cells. Thus, for example, the horizontal cells that express PLC β4 (14) and the Gq-coupling mGluR5 receptor (25) or interplexiform cells that release dopamine (26) could modulate rod function via a feedback signal, which is lost in the mutants. Another interesting alternative is that PLC β4 may be involved in regulating calcium in the subretinal space or extracellular resistance. An elevation of internal calcium in the rod in situ would lead to partial suppression of the rod circulating current by inhibiting guanylyl cyclase activity; this in turn would hyperpolarize the rods and partially activate rod-bipolars tonically, reducing the rod b-wave. Furthermore, effects on signaling in specific regions of the brain where PLC β4 is found to be localized could effect processing and integration of visual signals and thus elicit behavioral effects.
Acknowledgments
We thank Yanping Wu, Jeannie Chen, Jun Xu, Jason Chen, and Shirley Pease for their generous help. This work is supported by grants from the National Institutes of Health to M.I.S. (AG 12288) and to D.W. (GM 53162).
Footnotes
Abbreviations: PLC, phospholipase C; CS, conditioned stimulus; ERG, electroretinogram; US, unconditioned stimulus.
References
- 1.Gilman A G. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 2.Birnbaumer L, Abramowitz J, Brown A M. Biochim Biophys Acta. 1990;90:163–224. doi: 10.1016/0304-4157(90)90007-y. [DOI] [PubMed] [Google Scholar]
- 3.Berridge M J. Nature (London) 1989;341:197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- 4.Smrcka A, Hepler J, Brown K, Sternweis P. Science. 1991;251:804–807. doi: 10.1126/science.1846707. [DOI] [PubMed] [Google Scholar]
- 5.Taylor S, Chae H, Rhee S, Exton J. Nature (London) 1991;350:516–518. doi: 10.1038/350516a0. [DOI] [PubMed] [Google Scholar]
- 6.Wu D, Lee C-H, Rhee S G, Simon M I. J Biol Chem. 1992;267:1811–1817. [PubMed] [Google Scholar]
- 7.Wu D, Katz A, Lee C-H, Jiang H, Simon M I. J Biol Chem. 1992;267:25798–25802. [PubMed] [Google Scholar]
- 8.Wu D, Jiang H, Katz A, Simon M I. J Biol Chem. 1993;268:3704–3709. [PubMed] [Google Scholar]
- 9.Wu D, Katz A, Simon M I. Proc Natl Acad Sci USA. 1993;90:5297–5301. doi: 10.1073/pnas.90.11.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jhon D-Y, Lee H-H, Park D, Lee C-W, Lee K-H, Yoo O J, Rhee S-G. J Biol Chem. 1993;268:6654–6661. [PubMed] [Google Scholar]
- 11.Jiang H-J, Wu D, Simon M I. J Biol Chem. 1994;269:7593–7596. [PubMed] [Google Scholar]
- 12.Lee C W, Lee K H, Lee S B, Park D, Rhee S G. J Biol Chem. 1994;269:25335–25338. [PubMed] [Google Scholar]
- 13.Lee C W, Park D J, Lee K H, Kim C G, Rhee S-G. J Biol Chem. 1993;268:21318–21327. [PubMed] [Google Scholar]
- 14.Ferreira P A, Pak W L. J Biol Chem. 1994;269:3129–3131. [PubMed] [Google Scholar]
- 15.Min D S, Kim D, Seo J, Suh P, Ryu S H. J Biol Chem. 1993;268:12207–12212. [PubMed] [Google Scholar]
- 16.Bloomquist B T, Shortridge R D, Schneuwly S, Perdew M, Montell C, Steller H, Rubin G, Pak W L. Cell. 1988;54:723–733. doi: 10.1016/s0092-8674(88)80017-5. [DOI] [PubMed] [Google Scholar]
- 17.DeVries S H, Baylor D A. Cell. 1993;72:139–149. doi: 10.1016/s0092-8674(05)80033-9. [DOI] [PubMed] [Google Scholar]
- 18.Masu M, Iwakabe H, Tagawa Y, Yamashita M, Fukuda Y, Sasaki H, Nakamura Y, Takada M, Nakoa K, Katsuki M, Nakanishi S. Cell. 1995;80:757–765. doi: 10.1016/0092-8674(95)90354-2. [DOI] [PubMed] [Google Scholar]
- 19.Lyubarsky A L, Pugh E N. J Neurosci. 1996;16:563–571. doi: 10.1523/JNEUROSCI.16-02-00563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baylor D, Lamb P, Yau K Y. J Physiol (London) 1979;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Makino C L, Peachey N S, Baylor D A, Simon M I. Science. 1995;267:374–377. doi: 10.1126/science.7824934. [DOI] [PubMed] [Google Scholar]
- 22.Rhee S-G, Choi K D. J Biol Chem. 1992;264:12393–12396. [PubMed] [Google Scholar]
- 23.Robson J G, Frishman L. Visual Neurosci. 1995;12:837–850. doi: 10.1017/s0952523800009408. [DOI] [PubMed] [Google Scholar]
- 24.Gurevich L, Slaughter M. Vision Res. 1993;33:2431–2435. doi: 10.1016/0042-6989(93)90122-d. [DOI] [PubMed] [Google Scholar]
- 25.Hartvelt E, Brandstatter R, Wassle H. Eur J Neurosci. 1995;7:1472–1483. doi: 10.1111/j.1460-9568.1995.tb01142.x. [DOI] [PubMed] [Google Scholar]
- 26.Shulman L M, Fox D A. Proc Natl Acad Sci USA. 1996;93:8034–8039. doi: 10.1073/pnas.93.15.8034. [DOI] [PMC free article] [PubMed] [Google Scholar]


