Assisted conception technology has led to a variety of new techniques that can help subfertile couples. However, many now go beyond simply improving the capacity to procreate. They also affect areas outside reproductive biology and present new ethical dilemmas.
Figure 1.
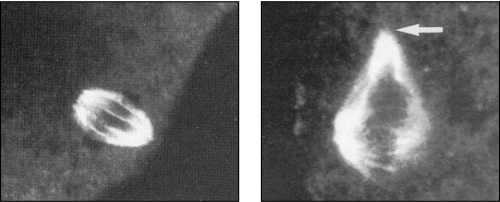
During cooling of an oocyte for cryopreservation, the normal metaphase spindle (left) can become dismantled (right), which could result in the production after fertilisation of a karyotypically abnormal embryo
Preserving fertility for young women
Long term survival rates for cancer have improved substantially because of the use of aggressive chemotherapy and radiotherapy. However, in young women this comes at a price—many women lose ovarian function because oocytes or their support cells are damaged by the treatment. A technique ensuring successful cryopreservation of oocytes would benefit women recently diagnosed with cancer who want to retain their fertility potential. In addition, cryopreservation could help women with a family history of premature menopause as they could store their gametes before their pool of oocytes is depleted.
Cryopreservation of oocytes
In contrast to the success of embryo freezing, which is now a routine procedure in most in vitro fertilisation clinics, cryopreservation of oocytes has been less successful. Only a few live births after egg freezing have been achieved since the first one in 1986. Some problems have been reduced by improving cryoprotectant regimens and by using intracytoplasmic sperm injection to overcome the block to fertilisation, resulting in a few pregnancies and live births. However, the success rate remains low—about 1 in 100 eggs that are frozen results in a live birth.
Figure 2.

Live births after egg freezing are rare and can make headline news
Cryopreservation of ovarian tissue and maturation of oocytes and follicles in vitro
An alternative approach is cryopreservation of slices or biopsies of ovarian tissue, which contain many thousands of immature oocytes. These oocytes are quiescent and their chromatin is in a stable phase of meiosis.
Autografting and in vitro maturation could be used to recover frozen oocytes for later use, but both methods are still experimental.
Autografting
Thawed slices of ovary might be grafted to the host, either to the remaining ovarian site or to an ectopic site such as the uterus or under the skin. With this technique, live births have been achieved in marmosets, sheep, mice, and recently in monkeys. To date, the only publicised attempt at human ovarian autotransplantation after cryopreservation was unsuccessful because folliculogenesis returned only for a short time. Indeed, preliminary experiments show that few oocytes survive in the tissue after grafting—sometimes the stored tissue is from patients with haematological or other malignancies with a propensity to metastasise, and so the safety of regrafting has been questioned.
Figure 3.
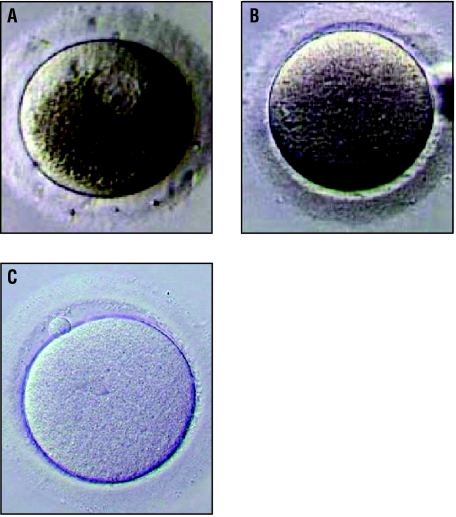
In in vitro maturation the germinal vesicle (within granular cytoplasm) of the immature oocyte (A) breaks down and the oocyte grows into a metaphase I oocyte (B). The egg becomes fully mature (metaphase II) when a polar body is extruded (C)
In vitro maturation
Finding a reliable protocol for the in vitro maturation of immature follicles is a major challenge. Complete in vitro development of immature follicles has been achieved in mice, but culture of ovarian tissue from large mammals and humans is difficult because normal oocyte growth is often compromised. The current dilemma is whether young women undergoing chemical or surgical oophorectomy should take the precaution of having some ovarian tissue frozen. Only if today's cryopreservation techniques are appropriate would they be able to use the stored tissue in future for in vitro maturation (when these methods are developed).
A major challenge in the cryopreservation of oocytes is to (a) preserve the egg's ability to be fertilised and (b) maintain the integrity of its genetic material so that a genetically normal embryo is produced
Preserving fertility in prepubertal boys
Testicular tissue from prepubertal boys (before Tanner stage 2) does not contain mature spermatozoa and so cannot be used for assisted reproductive techniques without maturation in vitro. As it is not possible to collect an ejaculated sample, cryopreservation of surgically retrieved testicular tissue may be the only option. Besides the practical difficulties yet to be overcome in the use of immature sperm, retrieval of such tissue presents legal and ethical dilemmas because the child is unlikely to be old enough to give informed consent. The Royal College of Obstetricians and Gynaecologists and the British Fertility Society have produced guidelines for storage and use.
Figure 4.
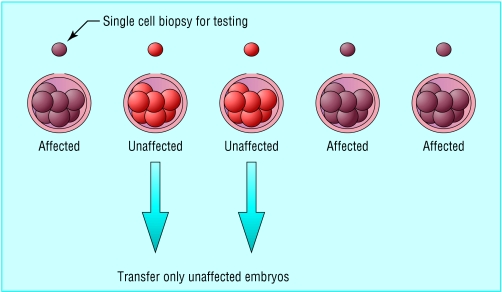
The principle of preimplantation genetic diagnosis. A single blastomere is removed from each 8-cell human embryo for the purposes of preimplantation genetic diagnosis. Up to two embryos found to be unaffected by the genetic disorder are transferred to the uterus
Preimplantation genetic diagnosis
Preimplantation genetic diagnosis (PGD) is an early alternative to prenatal diagnosis and is suitable for patients who are at substantial risk of conceiving a pregnancy affected by a known genetic defect. The technique has been applied to the analysis of numerical and structural chromosomal abnormalities that can result in handicap or recurrent miscarriage, the identification of sex to prevent transmission of X linked disease, and for the detection of specific serious monogenic disorders.
Table 1.
Preserving men's fertility
| • Testicular function is often compromised after chemotherapy treatment in much the same way as described above for ovarian tissue |
| • Preservation of fertility for men is much more straightforward than for women |
| • Freezing of multiple samples of ejaculated semen before chemotherapy and radiotherapy is successful |
| • Men about to have chemotherapy should be offered cryostorage of sperm as good practice |
For PGD, one or two cells are removed from embryos at the early cleavage stage and the diagnostic test is carried out on these cells. The genetic status of the embryo is inferred from result of the test, and only unaffected embryos are placed in the uterus. For single gene disorders, such as cystic fibrosis and spinal muscular atrophy, the polymerase chain reaction is used to amplify the region of the DNA containing the genetic lesion to levels where a diagnostic test can be carried out.
Figure 5.
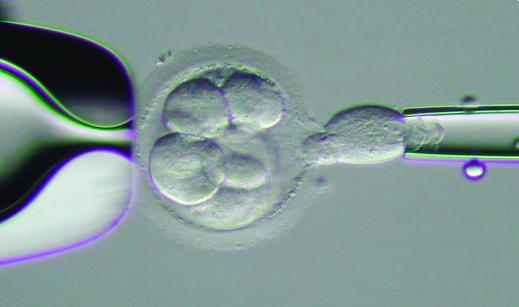
A single cell being removed from an 8-cell human embryo in vitro for genetic testing
PGD of sex linked diseases for which the specific genetic defect is unknown or not amenable to molecular diagnosis at the single cell level can be done using fluorescence in situ hybridisation (FISH). Probes that bind to specific chromosomal telomeres can be used to identify balanced or unbalanced products in Robertsonian and reciprocal translocations.
PGD is a highly specialised procedure that is available at only a few reproductive medicine centres worldwide, and the number of live births achieved is still relatively small. However, the use of this technique will expand rapidly as the molecular basis for more diseases is found.
Preimplantation screening of embryos
Implanting multiple embryos has led to an unacceptably high rate of multiple births. As the aim of assisted conception is to produce a healthy baby, reducing multiple pregnancy rates is a major goal and transferring a single embryo is the ideal. The ability to identify embryos with high implantation potential will allow more effective selection of embryos for embryo transfer and reduce the likelihood of multiple pregnancy without reducing overall pregnancy rates.
Figure 6.
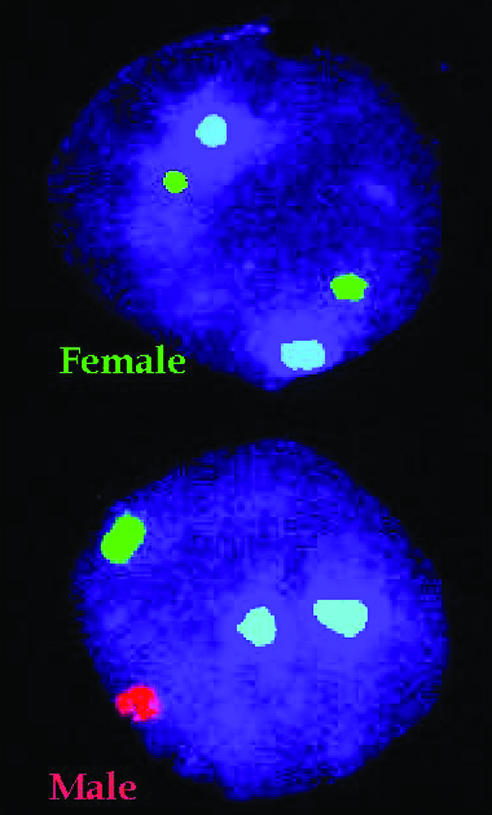
Preimplantation diagnosis using fluorescence in situ hybridisation (FISH). Specific probes, which bind to either X (green) or Y (red) chromosomes in the interphase nucleus fluoresce under ultraviolet illumination are used to determine the sex of the embryo
Selection of embryos for transfer is generally made on morphological grounds. Early cleavage stage embryos graded as high quality seem to have an improved implantation potential, although specificity is poor.
Some women, including those in older age groups (> 38 years) and those who have had repeated failure of in vitro fertilisation, are more likely to produce cytogenetically abnormal embryos, which are not capable of normal development. For these patients, preimplantation genetic screening or aneuploidy screening has been advocated. By using techniques developed for PGD to select normal embryos, the chromosomes that are responsible for the major survivable aneuploidies (for example, 21, 22, 18, 13, X, and Y) can be examined from an embryo biopsy using fluorescence in situ hybridisation. As over half of all embryos can be cytogenetically abnormal, excluding these embryos from selection has improved ongoing implantation rates in some studies. However, the restriction to the common aneuploidies still allows those embryos that may have other chromosomal rearrangements to remain undetected. This limits the usefulness of the screening.
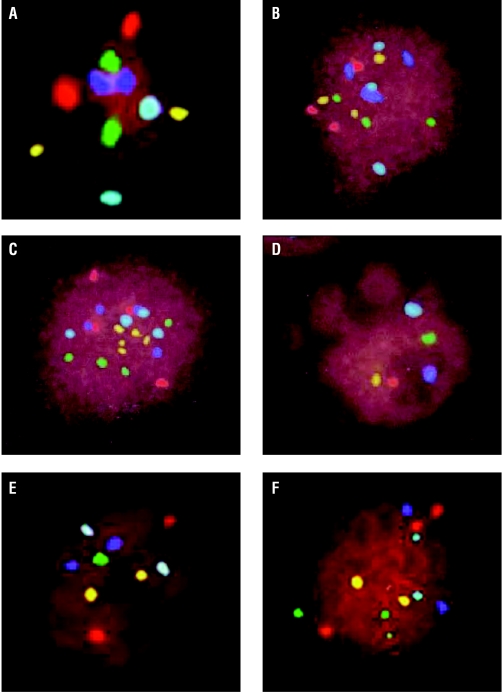
Figure 7.
Aneuploidy screening—normal embryos are diploid and have two spots for each colour (A). Many different abnormalities have been seen; some are shown above. The complete set of chromosomes has been duplicated in triploid (B) and tetraploid (C) nuclei. A set of chromosomes is missing in haploid embryos (D). Abnormalities of individual chromosomes are also seen (E), such as monosomy, trisomy, and double trisomy (F). If embryos with such abnormalities could be screened out, a higher implantation rate might be achieved after in vitro fertilisation
Stem cells and therapeutic cloning
Much media hype has surrounded the prospects of using embryonic stem cells in the treatment of degenerative diseases and the in vivo repair of damaged tissues. Potential treatments range from restoration of spinal cord function after injury to the cure of diabetes by replenishment of insulin producing cells of the pancreas. Neurological, hepatic, and cardiac or skeletal muscle cell lines derived from mouse embryos have all been used successfully in several mouse model systems to ameliorate symptoms previously untreatable by conventional treatment. However, derivation of embryonic stem cell lines in humans is more difficult. Many problems still have to be overcome before there is any prospect of their use in treatment.
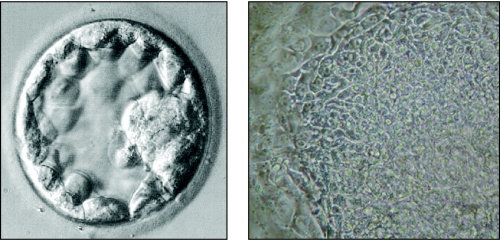
Figure 8.
Left: Blastocyst with outer layer of trophectoderm and clear inner cell mass. Right: Human embryonic stem cells derived originally from the inner cell mass
In the United Kingdom, after heated debate in both Houses and following the advice of a select committee of the House of Lords, legislation has been passed that allows research using human stem cells derived from human embryos that are surplus to in vitro fertilisation programmes. Creation of stem cell lines tailored to be immunologically compatible by cell nuclear replacement (therapeutic cloning) is also permitted, as is the creation of embryos specifically for this purpose. However, creation and use of human embryos in vitro falls under the Human Fertilisation and Embryology Act, and the Human Fertilisation and Embryology Authority (HFEA) will examine all requests. It is a requirement of an HFEA licence that a sample of the line is lodged with the newly created stem cell bank of the Medical Research Council, which will administer the use of the deposited cell lines by third parties.
Susan Pickering is senior lecturer in human reproductive biology in the department of women's health, Guy's, King's, and St Thomas's School of Medicine and scientific director of Guy's and St Thomas's assisted conception unit, London
The ABC of subfertility is edited by Peter Braude, professor and head of department of women's health, Guy's, King's, and St Thomas's School of Medicine, London, and Alison Taylor, consultant in reproductive medicine and director of the Guy's and St Thomas's assisted conception unit. The series will be published as a book in the winter.
Competing interests: None declared.
Further reading and resources
- • Chief Medical Officer's Expert Group on Therapeutic Cloning. Stem cells: medical progress with responsibility. DoH, 2000 www.doh.gov.uk/cegc/stemcellreport.htm [DOI] [PubMed]
- • Stem cell research report of House of Lords select committee. Stationery Office, 2002 www.publications.parliament.uk/pa/ld/ldstem.htm
- • Royal College of Obstetricians and Gynaecologists Working Party. Storage of ovarian and prepubertal testicular tissue. London: RCOG, 2000
- • Multidisciplinary Working Group of the British Fertility Society. A strategy for fertility services for survivors of childhood cancer. Hum Fertil 2003;6: 1-40 [Google Scholar]
- • Braude P, Pickering S, Flinter F, Mackie Ogilvie C. Preimplantation genetic diagnosis. Nat Gen Rev 2002:3: 941-53 [DOI] [PubMed] [Google Scholar]
- • Flinter F. Preimplantation diagnosis. BMJ 2001;322: 1008-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- • Department of Health. Preimplantation genetic diagnosis (PGD)—guiding principles for commissioners of NHS services. London: DoH, 2002