Abstract
Dinosaur dentine exhibits growth lines that are tens of micrometers in width. These laminations are homologous to incremental lines of von Ebner found in extant mammal and crocodilian teeth (i.e., those of amniotes). The lines likely reflect daily dentine formation, and they were used to infer tooth development and replacement rates. In general, dinosaur tooth formation rates negatively correlated with tooth size. Theropod tooth replacement rates negatively correlated with tooth size, which was due to limitations in the dentine formation rates of their odontoblasts. Derived ceratopsian and hadrosaurian dinosaurs retained relatively rapid tooth replacement rates through ontogeny. The evolution of dental batteries in hadrosaurs and ceratopsians can be explained by dentine formation constraints and rapid tooth wear. In combination with counts of shed dinosaur teeth, tooth replacement rate data can be used to assess population demographics of Mesozoic ecosystems. Finally, it is of historic importance to note that Richard Owen appears to have been the first to observe incremental lines of von Ebner in dinosaurs more than 150 years ago.
Keywords: dentine/histology/evolution/Crocodylia/Richard Owen
Growth lines in dinosaur dentine were first illustrated in the mid-1800s by the great British anatomist Richard Owen (1). Complementary discovery and descriptions of dinosaur growth lines were made later by Johnston (2). During an examination of thin sectioned dinosaur teeth, I observed several types of these laminations, the most conspicuous being ≈10–20 μm wide (Fig. 1). Subsequent thin sectioning and examination of teeth from extant mammalian and crocodilian taxa revealed growth bands of similar size and morphology (Fig. 1). In extant amniotes, such laminations are known as incremental lines of von Ebner (3–5). They form daily in most taxa (3–10)—the plesiomorphic rate for the clade (6). Because dentine deposition occurs throughout tooth formation, counts of cyclically deposited growth lines can be used to infer tooth development times, providing the rate of formation can be ascertained (6, 11, 12). Furthermore, growth line counts can be used to infer tooth replacement rates in taxa with polyphyodont (i.e., continual) tooth replacement (6). I have demonstrated, using periodic chemical labeling, that incremental lines of von Ebner form daily in crocodilians (6), the closest living outgroup to the Dinosauria and morphologically the most suitable model for studying dinosaur tooth formation. Thus, if incremental lines of von Ebner exist in dinosaur dentine, it can be inferred on phylogenetic grounds that they formed daily. In this study, I report confirmation of the small laminations in dinosaur dentine as incremental lines of von Ebner and use line counts to estimate the formation and replacement rates of dinosaur teeth. These data can be used to augment our understanding of the evolution and functional morphology of dinosaur dentitions. Finally, I suggest that population demographics of Mesozoic ecosystems can be reconstructed using tooth replacement rate data.
Figure 1.
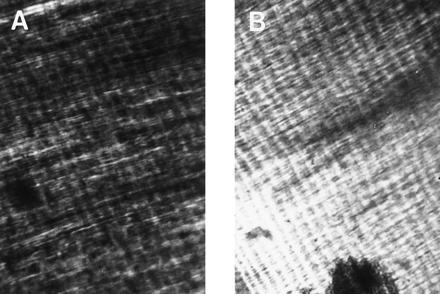
Comparison of mammalian incremental lines of von Ebner with dinosaur growth lines in thin sectioned teeth viewed with polarized light. (A) Human dentine. (B) Edmontonia dentine. The incremental lines run from upper left to lower right in each plate and consist of alternating dark and light bands (≈25 are visible in each plate). The spacing interval of the incremental lines is ≈15 μm. Note that the dinosaur specimen exhibits the von Ebner features of alternating broad opaque zones and finer transparent laminae, dentinal tubules (thin, fibrous structures running from lower left to upper right in the plates) intersecting the growth lines at right angles, and increment widths between 1 and 30 μm.
MATERIALS AND METHODS
Specimens.
Intact dentitions and shed teeth of 26 dinosaurs representing 10 genera from the Late Cretaceous Cloverly, Two Medicine, Judith River, Prince Creek, and Hell Creek formations of Montana and Alaska were selected for histologic examination (Table 1). Teeth shed by crocodilians that lived contemporaneously with the dinosaurs from the Cloverly and Hell Creek formations were also examined. All specimens belong to the Museum of the Rockies, Montana State University, Bozeman. Thin sections are housed in the museum’s Comparative Histology Collection.
Table 1.
Tooth sizes, von Ebner incremental line widths, tooth formation, and replacement rate estimates for fossil archosaurs
| Taxon | Sample size, n | Mean crown volume, ml | Increment size range, μm | Mean increment width, μm | Mean tooth formation rate, days | Tooth replacement rate, days |
|---|---|---|---|---|---|---|
| Crocodilian† | 1 | 0.90 | 8–19 | 13.0 | 246 | NA |
| Leidyosuchus† | 1 | 1.20 | 13–22 | 19.0 | 283 | NA |
| Adult Edmontonia‡ | 1 | 0.20 | 9–17 | 13.5 | 279 | NA |
| Adult Triceratops§ | 2 | 2.65 | 13–26 | 15.8 | 381 | 83 |
| Infant Lambeosaurinae¶ | 5 | 0.12 | 11 | 11.0 | 147 | 60 |
| Adult Prosaurolophus¶ | 3 | 2.0 | 15–28 | 16.0 | 323 | 81 |
| Infant Maiasaura¶ | 1 | 0.10 | 11–19 | 12.0 | 132 | 46 |
| Adult Maiasaura¶ | 1 | 1.90 | 13 | 13.0 | 281 | 58 |
| Juvenile Edmontosaurus¶ | 3 | 0.43 | 12–21 | 14.0 | 225 | NA |
| Adult Edmontosaurus¶ | 2 | 2.0 | 11–25 | 19.8 | 339 | 50 |
| Adult Deinonychus** | 1 | 0.20 | 6–12 | 10.1 | 413 | 290* |
| Adult Troodon‡‡ | 2 | 0.04 | 7–15 | 11.0 | 363 | NA |
| Juvenile Albertosaur†† | 1 | 1.80 | 12–17 | 14.5 | 339 | 296* |
| Adult Albertosaur†† | 1 | 18.0 | 12–24 | 14.0 | 519 | 454* |
| Juvenile Tyrannosaurus†† | 1 | 1.80 | 12–17 | 14.0 | 264 | NA |
| Sub. Ad. Tyrannosaurus†† | 1 | 15.5 | 8–17 | 14.0 | 314 | NA |
| Adult Tyrannosaurus†† | 1 | 138 | 7–22 | 17.0 | 933 | 777* |
Selection of Mean-Sized Teeth.
In extant crocodilians, teeth of mean size (by volume) within an individual’s dentition are replaced at rates approximating the mean rate for the entire dentition (6). Thus, to allow estimations of tooth formation and replacement rates using incremental line counts, teeth of mean size in each dinosaur dentition (hereafter “representative” teeth) were identified. Representative teeth were recognized by molding the functional tooth crowns with dental putty and casting them in epoxy. The casts were immersed in a water-filled graduated cylinder, and the volumetric displacement was ascertained (6). The volumes of shed teeth were also determined using this method.
Thin Sectioning.
One representative tooth family [a functional tooth and its respective developing replacement teeth, sensu (18)] was removed from each of the intact dentitions and prepared for histologic examination. The jawbone bounding a tooth family anteriorly and posteriorly was sawed in the frontal plane using a slow speed bone saw fitted with a diamond-tipped wafering blade (6). Each tooth family and attending alveolar bone were embedded in clear plastic. A thin section 1.0–3.0 mm in width was made in the frontal plane through the coronal end of each tooth in the tooth families. The thin sections were mounted on glass slides and sanded to a 50- to 100-μm thickness using descending grits of silica-carbide sandpaper (60–600 grit) and polished on a felt pad with wetted aluminum oxide powder. For several specimens (Table 1), it was not possible to section the jaws because of the rarity of the specimens. Consequently, the development and replacement rates were assessed using an alternative method involving computerized tomography (see below).
Growth Line Verification and Identification.
If the teeth of the fossil crocodilians (i) possessed comparable growth lines to those in dinosaur teeth and (ii) these growth lines were morphologically equivalent to those in extant crocodilians, it would suggest that the fossil crocodilian and dinosaur laminations were the result of a biologic process and not a product of diagenesis. Consequently, I characterized the morphology of the incremental lines in the dinosaur teeth in terms of histologic structure, depositional plane trajectories, and widths. These attributes were contrasted with those features of the incremental lines in fossil and extant crocodilians (6). All teeth were viewed using polarized microscopy at a magnification of ×40–100. Width assessments were made by projecting images of the thin sections onto a high resolution video screen. The growth lines were measured perpendicular to the depositional front (6) using an image analysis program (IM 300A version 3.0, Analytical Imaging Concepts, Irvine, CA). Five counts were made for each tooth. To test whether the dinosaur growth bands were incremental lines of von Ebner, their morphologic characteristics were contrasted with incremental lines of von Ebner in teeth of extant mammals and crocodilians.
Incremental Line Counts.
The total number of growth lines in each thin sectioned tooth was determined. For the biphid-rooted Triceratops horridus teeth (18), dentine had been deposited on both the crown and root surfaces simultaneously (personal observation). Incremental line counts were made using the coronal dentine.
Calculating Tooth Replacement Rates.
To ascertain representative tooth replacement rates (presumably in days), the total number of incremental lines present in each functional tooth was subtracted from those in its immediate successor (ref. 6; Fig. 2). [In hadrosaur and ceratopsian dentitions, the teeth occupying the antepenultimate and penultimate positions within each tooth family were fully formed before partition (unpublished observation). Consequently, incremental line counts were made between successive, incompletely developed teeth nearer the dental lamina in each tooth family.] These rate estimates presumably approximate the mean tooth replacement rates for the dentitions from which they were extracted (6).
Figure 2.
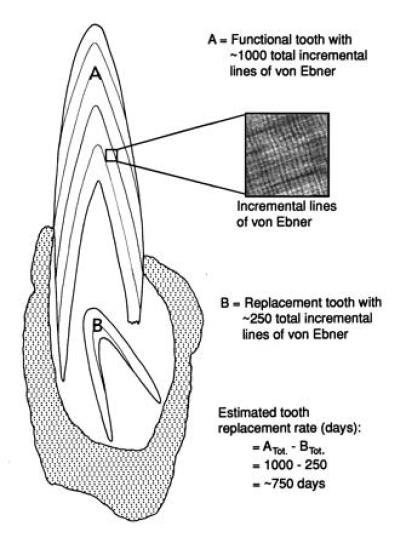
Theoretical assessment of the tooth replacement rate for a representative tooth family of a dinosaur (T. rex) thin sectioned in the frontal plane. The total number of incremental lines in a thin sectioned functional tooth (A) is subtracted from the number in its respective replacement (B). This calculation gives a temporal approximation of how far ahead developmentally the functional tooth is relative to the replacement tooth and thus gives a replacement rate estimate for the tooth position. Because representative teeth are of mean size (by volume) for the dentition from which they were extracted, these estimates approximate the mean rates for the dentition. This method has been tested on crocodilians (Alligator mississippiensis), the extant outgroup to the Dinosauria and most suitable model for dinosaur tooth formation and replacement (6).
To estimate the tooth replacement rates for the dinosaur specimens for which it was not feasible to thin section the intact jaws, an alternate means of assessment was used. First, the mean increment width was determined for an associated tooth of representative size from each dentition. Then the jaws and individual tooth families were scanned in the frontal plane by computerized tomography. Using full scale photos of a scanned representative tooth family, the amount of dentine (measured perpendicular to the incremental line trajectories) in both the functional and replacement teeth was determined. These measures were divided by the mean incremental line width (obtained from the individual associated teeth, see above) to determine tooth formation times (presumably in days). To estimate the mean tooth replacement rates, the replacement tooth formation rates were subtracted from the functional tooth values (6).
RESULTS
When viewed with polarized microscopy, all thin sectioned fossil crocodile and dinosaur teeth exhibited dentine growth lines (Figs. 3 and 4); see ref. 2. These laminations were morphologically comparable to the incremental lines of von Ebner of extant mammalian and crocodilian teeth (Figs. 1 and 4). They exhibited the characteristic von Ebner features of alternating opaque zones and transparent laminae in polarized light, line trajectories paralleling the tooth crown border, widths ranging between 1 and 30 μm, and dentinal tubules intersecting the growth lines at right angles (3–5). Signs of pathologically disrupted odontogenesis, such as the deposition of interglobular dentine (3–4) or broadly folded dentinal tubules (3–5), were not found in any of the fossil teeth.
Figure 3.
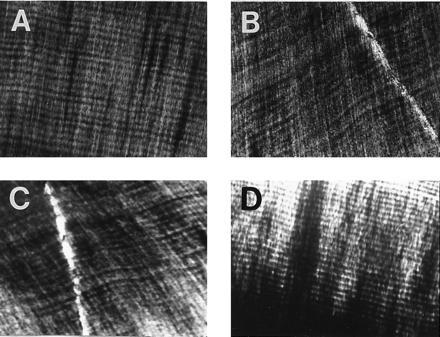
Incremental lines in the dentine of representatives from various dinosaur clades. The incremental lines of von Ebner run from left to right in each plate and are the smallest visible laminations. The teeth were thin sectioned longitudinally and viewed with polarized microscopy. (A) Tyrannosaurus (Tyrannosauridae); (B) Triceratops (Ceratopsidae); (C) Edmontosaurus (Hadrosauridae); (D) Edmontonia (Nodosauridae). The spacing interval of the incremental lines is approximately 15 μm.
Figure 4.
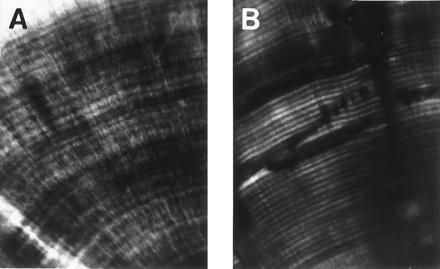
Comparison of fossil crocodilian (Neosuchia) incremental lines with incremental lines of von Ebner in extant crocodilian (A. mississippiensis) dentine. The specimens were thin sectioned transversely and viewed with polarized microscopy. The spacing interval of the incremental lines is approximately 13 μm.
Measurements of the dinosaur incremental line widths revealed a range of 6–28 μm and mean values ranging from 10.1 to 19.8 μm (Table 1). There was a general increase in incremental line widths through ontogeny in the taxa for which multiple age specimens were available (Table 1). Estimates of tooth formation rates ranged from 132 to 933 days (Table 1). In general, larger toothed individuals had slower tooth formation rates (Table 1).
Estimated tooth replacement rates for the dinosaurs ranged from 46 to 777 days (Table 1). Within the Theropoda, the larger toothed taxa exhibited the slowest rates of tooth replacement (Table 1). Tooth replacement rates slowed through ontogeny in the tyrannosaurids and in Maiasaura peebelesorum (Table 1).
DISCUSSION
This study shows that von Ebner-like incremental lines are prevalent in the dentine of Mesozoic dinosaurs and crocodilians (see refs. 1 and 2). Because comparable laminations also form during odontogenesis in extant crocodilians (refs. 6, 19, 20; Fig. 4), I concluded that these laminations were a product of a physiologic process and not fossilization.
The dinosaur laminations are morphologically similar to incremental lines of von Ebner in extant amniotes, including crocodilians (the extant outgroup to the Dinosauria), so I suggest that their similarities reflect homology. Furthermore, because incremental lines of von Ebner form daily in most amniotes, including crocodilians, it is likely that the von Ebner lines in dinosaurs formed daily as well.
Barring pathological, metabolic disruptions of odontogenesis, dentine is formed continually from tooth inception to completion in extant amniotes (3, 4). Because indicators of traumatic disruption were not found in the dinosaur dentine, it is likely that the formation rhythm was maintained throughout tooth development. Consequently, it is reasonable to assume that incremental line counts can be used reliably to infer tooth development times and replacement rates for these specimens (6).
The tooth replacement rate estimates for the theropod dinosaurs exhibited slowing with increased tooth size, both intraspecifically and interspecifically. Slowing of rates with size is characteristic of polyphyodont gnathostomes for it is also exhibited by extant lepidosaurian (21) and archosaurian reptiles (6, 22) and “fishes” (e.g., chondrichthyans; see ref. 23).
The neoceratopsian and hadrosaurian dinosaurs had relatively more rapid rates of tooth replacement than theropodan taxa for any given tooth size. In addition, unlike theropods and most other gnathostomes (6, 21–23), they retained relatively rapid rates of tooth replacement despite substantial increases in tooth size throughout ontogeny.
Dentine formation rates (and incremental line widths) in amniotes are between 1 and 30 μm per day (e.g., refs. 6, 7, 10, and 24). This rate holds true for the minuscule teeth of rodents (9) and the comparatively enormous tusks and molars of proboscidians (unpublished data; also see refs. 19 and 25). In crocodilians, daily dentine formation rates (and the corresponding incremental line widths) increase through ontogeny. However, the rates plateau with increased body size and normally do not exceed 30 μm/day (unpublished data; ref. 6). Considering these results, I suggest that there is a limitation to the amount of dentine formed on a daily basis in amniote teeth. [Mammalian enamel similarly has a limited range of formation rates (26).] Whether this constraint is structural or physiologically controlled is indeterminable at present. Nevertheless, it appears to have affected the formation of the incremental lines in dinosaurs. Despite the 3500-fold volumetric range in dinosaur tooth sizes surveyed, the incremental line widths (and presumably the daily dentine formation rates) all fell within a 1- to 30-μm range. This constraint, when viewed in conjunction with tooth formation and replacement rate data, has important ramifications for understanding the evolution and form/function relationships of dinosaur dentitions. For example, during the evolution of the tyrannosaur clade, gigantism was attained. As body sizes increased, so did tooth size. However, the time required to develop and replace teeth slowed as larger tooth sizes were attained and dentine formation rates plateaued. As a consequence, the teeth of larger derived forms, like those of Tyrannosaurus rex, required several years to form and be replaced. Although larger size certainly increased the size of prey items that could be procured by tyrannosaurs, the risk of long term (months to years) debilitation after dental trauma also increased. Natural selection appears to have compensated for this trade-off by diminishing the likelihood of dental injuries in this lineage via allometric growth. Relative to the plesiomorphic coelurosaurian condition (17), tyrannosaur teeth became more blunt-tipped (unpublished observations), and the crowns became more robust transversely (27). These changes made the dentition more impact-resistant, bolstered bending strength, and enhanced the overall durability and functional longevity of the teeth (27–29).
The evolution of hadrosaurian and ceratopsid dental batteries [a dental battery consists of dozens of tightly packed tooth families that effectively form one giant “tooth” with a unified wear surface (18)] can be explained by limitations in dentine deposition rates as well. These structures presumably evolved in response to a dietary shift to more abrasive or durable foodstuffs requiring mechanical processing before digestion (16). The consumption of these foodstuffs promoted the rapid attrition of the functional teeth. For instance, based on tooth crown heights (unpublished data) and replacement rate data (this study), infant M. peeblesorum tooth crowns wore down at 0.2 mm/day and adults at 0.5 mm/day. [These rates rival the fastest tooth wear rates in extant animals (0.19–0.99 mm/day; ref. 30)]. As abrasive plants increasingly were consumed by the respective ancestors of the hadrosaurs and ceratopsids, their tooth wear rates presumably increased to levels approaching the maximal output of the dentine-forming odontoblasts. Consequently, tooth longevity [as in the evolution of ungulates (31)] or dentine formation rates had to increase if this trend was to be continued. Natural selection largely favored the latter solution. Increased numbers of replacement teeth were added to each tooth family, thus increasing the number of layers of odontoblasts simultaneously producing dentine at each tooth position. These dinosaur lineages changed from the ancestral state of one to two replacement teeth to three to six (ref. 18; unpublished observations). Thus, with the evolution of tooth batteries, increases in potential dentine formation rates of 50–600% were gained.
Ontogenetic variations in the number of replacement teeth in tooth families may reflect wear rate variability as well. For example, M. peeblesorum had two replacement teeth as infants (unpublished observation) and four or five as adults (32). The approximate doubling of numbers of replacement teeth (and consequently the number of odontoblast layers secreting predentine) accommodated the approximate doubling of wear rates through ontogeny (see above), and thus a functional dentition was maintained throughout life.
In addition to adding replacement teeth to tooth families to increase the number of active odontoblasts per tooth position, the ceratopsids developed two odontoblast layers that simultaneously formed dentine within each tooth, one forming the crown and one the paired roots. This mechanism served to increase individual tooth formation rates relative to the plesiomorphic single-rooted (single odontoblast-layered) condition of basal ceratopsians (18).
Shed dinosaur teeth are one of the most commonly found entities in Mesozoic formations. By incorporating tooth replacement rates (from different taxa and ontogenetic stages) and counts of shed teeth from faunas, it may be possible to assess aspects of dinosaur population dynamics at the demal level. For example, age structuring, predator–prey ratios, and species diversity (sensu: ref. 33) might be ascertained. This method might be a better indicator of population demographics than making estimates based on skeletal counts (either whole skeletons or individual bones) because shed teeth are not subjected to taphonomic biases due to the activities of carnivorous taxa [e.g., bone consumption and skeletal scattering (6, 34)]. However, tooth counts are not completely unbiased because sorting can concentrate teeth of certain sizes or type (e.g., lag deposits) and diagenetic effects may preferentially denude weaker tooth types [e.g., unlike theropod teeth, hadrosaur and ceratopsian teeth are not fully enveloped by wear-resistant enamel (18)]. Nevertheless, the collection of teeth shed from certain geologic settings, such as flood plain or eolian deposits, might be considered largely free of such biases and might provide useful data.
The discovery (observation and description) of incremental lines of von Ebner is generally credited to von Ebner in 1906 (35); however recent reviews (e.g., refs. 36 and 37) suggest that Andreasen (38) was actually the first to note their existence several years earlier. It is of historic interest to point out that, although Owen did not allude to their presence in his texts from the 1840s (1), the plates from his dental research suggest that he was the first to observe incremental lines of von Ebner (e.g., plates 70AE and 71 in ref. 1). Whether Owen recognized the biologic significance of these laminations is open to conjecture.
Acknowledgments
I thank Marvalee Wake, John Horner and the Museum of the Rockies, Robert Moore, Harold Picton, James Wilson, David Wake, Kevin Padian, James Valentine, Katie Best, Kate Erickson, David Varricchio, Allison Gentry, Dianne Gabriel, Yoshihiro Katsura, Pat Leiggi, Mark Goodwin, Ellen Lamm, Karen Belinky, Joe Tkach, Terry Panasuk, Bob Harmon, Carrie Ancell, Philip Currie, Gayle Nelms, Renée Dickie, Meredith Mahoney, Karen Klitz, and Wendy Olson for their assistance and helpful input. The Bozeman Deaconess Hospital generously donated computerized tomography scans for this study. This research was funded by the Museum of Vertebrate Zoology of the University of California at Berkeley and a John D. and Catherine T. MacArthur Foundation Fellowship presented to John R. Horner.
References
- 1.Owen R. Odontography. London: Bailliere; 1840–1845. [Google Scholar]
- 2.Johnston P A. Nature (London) 1979;278:635–636. [Google Scholar]
- 3.Ten Cate A R. Oral Histology: Development, Structure and Function. St. Louis: Mosby; 1985. [Google Scholar]
- 4.Hillson S. Teeth. Cambridge, U.K.: Cambridge Univ. Press; 1986. [Google Scholar]
- 5.Avery J K. In: Orban’s Oral Histology and Embryology. Bhasker S N, editor. St. Louis: Mosby; 1991. pp. 106–138. [Google Scholar]
- 6.Erickson G M. J Morphol. 1996;228:189–194. doi: 10.1002/(SICI)1097-4687(199605)228:2<189::AID-JMOR7>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 7.Schour I, Hoffman M M. J Dent Res. 1939;18:161–175. [Google Scholar]
- 8.Yilmaz N H N, Newman H N, Poole D F C. Arch Oral Biol. 1977;22:511–513. doi: 10.1016/0003-9969(77)90046-2. [DOI] [PubMed] [Google Scholar]
- 9.Schour I, Steadman S R. Anat Rec. 1935;63:325–333. [Google Scholar]
- 10.Rosenberg G D, Simmons D J. Calcif Tissue Int. 1980;32:45–53. doi: 10.1007/BF02408520. [DOI] [PubMed] [Google Scholar]
- 11.Myrick A C., Jr Ann Zool Fenn. 1984;171:217–220. [Google Scholar]
- 12.Dean M C. Hum Evol. 1987;16:157–172. [Google Scholar]
- 13.Benton M J, Clark J M. In: Phylogeny and Classification of the Tetrapods: Amphibians, Reptiles and Birds. Benton M J, editor. Vol. 1. Oxford: Clarendon; 1988. pp. 295–338. [Google Scholar]
- 14.Coombs W P, Maryaska T. In: The Dinosauria. Weishampel D B, Dodson P, Osmolska H, editors. Berkeley, CA: Univ. Calif. Press; 1990. pp. 456–483. [Google Scholar]
- 15.Dodson P, Currie P J. In: The Dinosauria. Weishampel D B, Dodson P, Osmolska H, editors. Berkeley, CA: Univ. Calif.; 1990. pp. 593–618. [Google Scholar]
- 16.Weishampel D, Horner J R. In: The Dinosauria. Weishampel D B, Dodson P, Osmolska H, editors. Berkeley, CA: Univ. Calif.; 1990. pp. 534–561. [Google Scholar]
- 17.Holtz T R., Jr J Paleontol. 1994;68:1100–1117. [Google Scholar]
- 18.Edmund A G. Contrib Life Sci Div R Ont Mus. 1960;52:1–90. [Google Scholar]
- 19.Schour I, Hoffman M M. J Dent Res. 1939;18:91–102. [Google Scholar]
- 20.Schmidt W J, Keil A. Polarizing Microscopy of Dental Tissues. Oxford: Pergamon; 1971. [Google Scholar]
- 21.Kline L W, Cullum D. J Herpetol. 1984;18:176–185. [Google Scholar]
- 22.Poole D F G. Proc Zool Soc London. 1961;136:131–140. [Google Scholar]
- 23.Moss S A. In: Sharks, Skates, and Rays. Gilbert P W, Mathewson R F, Rall D P, editors. Baltimore: Johns Hopkins Univ. Press; 1967. pp. 319–329. [Google Scholar]
- 24.Hoffman M M, Schour I. Anat Rec. 1940;78:233–252. [Google Scholar]
- 25.Fisher D C. In: Late Pleistocene and Early Holocene Paleoecology and Archaeology of the Eastern Great Lakes Region. Laub R S, Miller N G, Steadman D W, editors. Vol. 33. Buffalo, NY: Buffalo Society of Natural Sciences; 1988. pp. 116–125. [Google Scholar]
- 26.Dumont E R. Scand Microsc Int. 1995;9:429–442. [PubMed] [Google Scholar]
- 27.Farlow J O, Brinkman D L, Abler W L, Currie P J. Mod Geol. 1991;16:161–198. [Google Scholar]
- 28.Abler W L. Paleobiology. 1992;18:161–183. [Google Scholar]
- 29.Erickson G M, Olson K H. J Vertebr Paleontol. 1996;16:175–178. [Google Scholar]
- 30.Howard W E, Smith M E. J Mammal. 1952;33:485–487. [Google Scholar]
- 31.Carroll R L. Vertebrate Paleontology and Evolution. New York: Freeman; 1988. [Google Scholar]
- 32.Horner J R. J Vertebr Paleontol. 1983;3:29–38. [Google Scholar]
- 33.Purves W K, Orians G H, Heller H G. Life: The Science of Biology. Salt Lake City: Freeman; 1992. [Google Scholar]
- 34.Shipman P. Life History of a Fossil. Cambridge, MA: Harvard Univ. Press; 1981. [Google Scholar]
- 35.von Ebner V. Sber Akad Wiss Weing. 1906;115:281–349. [Google Scholar]
- 36.Kawasaki K, Tanaka S, Ishikawa T. Arch Oral Biol. 1980;24:939–943. doi: 10.1016/0003-9969(79)90221-8. [DOI] [PubMed] [Google Scholar]
- 37.Ohtsuka M, Shinoda H. Arch Oral Biol. 1995;40:481–485. doi: 10.1016/0003-9969(95)00002-7. [DOI] [PubMed] [Google Scholar]
- 38.Andreasen V. Dtsch Mschr Zahnheilk. 1898;16:386–389. [Google Scholar]


