Abstract
The “shape” of a female mating preference is the relationship between a male trait and the probability of acceptance as a mating partner. The shape of preferences is important in many models of sexual selection, mate recognition, communication, and speciation, yet it has rarely been measured precisely. Here I examine preference shape for male calling song in a bushcricket (katydid). Preferences change dramatically between races of a species, from strongly directional to broadly stabilizing (but with a net directional effect). Preference shape generally matches the distribution of the male trait. This is compatible with a coevolutionary model of signal-preference evolution, although it does not rule out an alternative model, sensory exploitation. Preference shapes are shown to be genetic in origin.
Keywords: insect song, phonotaxis, premating reproductive isolation, speciation, sexual selection
Understanding the attributes of female mating preferences is essential for progress in the study of sexual selection and in the assessment of many models of animal speciation (1–4). The “shape” of a female mating preference is the relationship between a male trait (for example, tail length in many sexually dimorphic birds or the interpulse interval of the courtship song of Drosophila melanogaster) and the probability of acceptance of that trait value in a mating partner. It is the behavioral response of females to males, not to be confused with the individual sensitivities of sensory neural inputs. Typically, preference shapes are described as “stabilizing” (females favor typical trait values) or “directional” (females favor an extreme). These alternatives have important consequences for the evolutionary dynamics of sexual systems, because directional preferences can favor ambidirectional modifier genes (particularly if the shape is greater than linear) and, hence, indirectly cause higher-than-average genetic variation for sexually selected traits (5). Another area in which preference shape is important concerns the function of the sexual communication system. It is often supposed that stabilizing (or absolute) preferences suggest a species or specific-mate recognition function, whereas directional preferences are more likely to lie behind the exaggerated traits of sexual selection (4, 6–10). However, it is difficult to infer function from only the shape of a female mating preference (3, 10). For example, a normal preference function that is displaced from the male trait distribution will have a net effect of strongly directional selection. Hence, influential models of Fishers Runaway Process (11) have achieved exaggeration of male traits with a normal female preference function. Although the shape of the preference alone is not a reliable guide to its ultimate function, the preference function’s shape and degree of overlap with the male trait distribution will determine the extent to which sexual selection will arise in a communication system.
To date, little empirical work has directly addressed the shape of mating preferences (12), and almost all work has inferred the shape from binomial choice tests. For example, studies of frogs imply stabilizing preferences for important traits with some examples of directionality, especially for more complex traits (2). Mismatches between the most preferred trait and the mean trait have been found (13), but it is usually not possible to say what preference shape is responsible for this. An exception is Basolo’s study of Xiphophorus, which implied the displaced preference function was linear (14). The phonotaxis system of crickets allows multiple testing of the sexual preferences of females and has been used to infer attributes of female preference functions (12). I have given bushcrickets (katydids) the choice between two artificial songs. One song (not normally preferred by the female being tested) was held constant, and an important parameter of the other song was varied systematically but in random sequence. Variation in the preferences shown by females allows the shape of preference functions to be plotted.
MATERIALS AND METHODS
The study organism was the bushcricket Ephippiger, which has a typical tettigoniid mating system with males calling and females approaching to initiate mating (15). The song of Ephippiger ephippiger varies geographically, and “song races” have been defined on the basis of changes in the number of syllables per chirp (Fig. 1) (16, 17). Usually, E. ephippiger produces monosyllabic chirps, but around the eastern Pyrenees and Mediterranean coast, syllables per chirp increase to an average of four or five. In polysyllabic populations, mean syllable number per male is highly repeatable: 0.74 in the laboratory (18) and 0.60 when measured directly in the field (based on the analysis of 85 recordings of 30 marked individuals), i.e., much of the variation in the trait is distributed among rather than within males (19). This level of repeatability indicates that the trait can provide females with a reliable signal. The spectral profile of the song is peaked around 15 kHz, extends to over 40 kHz, and does not vary between song races. In addition to syllable number, the song of these races also varies in the number of stridulatory peg strikes in the closing movement of each syllable and in the peg strike rate (together determining syllable length).
Figure 1.
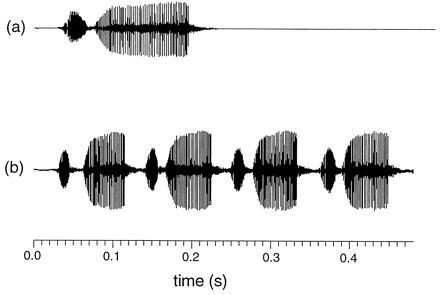
Chirps of calling song of monosyllabic (a) and polysyllabic (b) song races of Ephippiger. Each syllable consists of a short opening and longer closing wing movement (during which individual stridulatory peg strikes can be seen), and the major component for female preference is the longer closing movement.
Females prefer song typical of their own population (17, 18). Hybrid males have intermediate syllable numbers, and hybrid females prefer their song (20). I have constructed models of polysyllabic, monosyllabic, and hybrid song that vary in syllable number. Song synthesis was carried out using the signal software system (Engineering Design, Belmont, MA) at a digital-to-analog rate of 250 kHz, a synthetic opening syllable, and a single real stridulatory peg impact, recorded using a Beyer (Heilbronn, Germany) MCE 6.1 microphone. The major syllable was made by inserting the peg strike repeatedly into a file at a location specified by the experimenter to produce a syllable of an appropriate length and a peg strike rate for the song type. The amplitude envelope for syllable shape was the average of six extracted from different recordings. It and the opening syllable were invariant across song models. For playback, the models were recorded onto a two-track TEAC (Tokyo, Japan) reel-to-reel tape recorder (model X-2000M, tape speed 38.1 cms−1) and replayed through Ultra Sound Advice (London) ultrasonic amplifiers (S55) and speakers (S56) in a sound-attenuated room. Subtle details of the models are faithfully reproduced with this equipment (21).
Virgin females, reared in the laboratory, were given a choice between their native song and a non-native song. The native song was varied in syllable number, with each female given eight choices to each song pair. A single trial consisted of playing one song pair to a female four times, switching the song between speakers for consecutive presentations. Four choices of one song therefore requires three consecutive changes of direction. A complete set of song pairs was played to each female in random sequence, with a minimum of 20 min between each trial, then another set was completed with a different random sequence. Multiple testing under such circumstances does not influence the probability of a “correct” choice of song (personal observation), and furthermore, the random sequence presentation controls for sequence effects. All females completed all trials and are equally represented in the data, which were analyzed using cubic splines. These are appropriate when the shape of the function is the feature under study (22), and bootstrapping allows errors to be fitted to the curve. Although this technique was developed for a binomial response against a continuous variate, its use here is appropriate, because the discrete syllable numbers of Ephippiger reflect an underlying continuous trait (19) and the female preferences are likely to be continuous. Before fitting splines, a random error of ±2.5% was introduced to the syllable number to avoid ties (22). This influenced the spread of peaks in the curves, but to the same extent for all analyses. The smoothing factor was a constant −6, approximately the average of the best factors from independent analyses of the combined results for each female type.
All females used in this study were virgins from laboratory stocks of French origin. The Ephippiger terrestris stock was collected near the village of Seyne, département Alpes de Haute Provence. The E. ephippiger stocks were all of the “diurnus” complex (23). The polysyllabic race was collected near the village of Sauto, département Pyrénées Orientales, and the monosyllabic race was collected near Montpellier, département Hérault. The hybrids were from crosses between the same polysyllabic stock and a monosyllabic stock collected near Valensole, département Alpes de Haute Provence.
RESULTS
Fig. 2 shows the preferences of females of E. terrestris, a monomorphic monosyllabic species, and of a monosyllabic population of the polymorphic species E. ephippiger. Both are sharply peaked around one syllable per chirp, although it is notable that females of the monomorphic species have a “tighter” preference function than those of the polymorphic species (i.e., they are less likely to respond to a three- or four-syllabic chirp).
Figure 2.
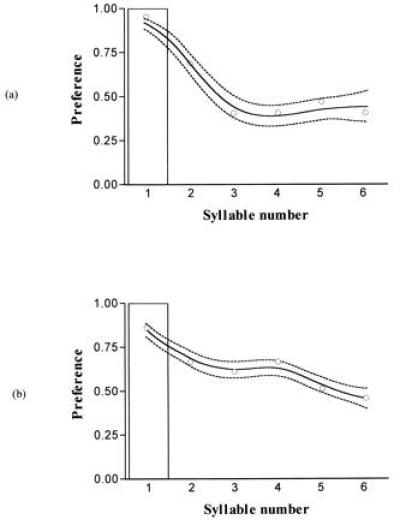
The shape of preference of monosyllabic females of Ephippiger. (a) Combined results of the analysis of four females of E. terrestris, which is monosyllabic throughout its range. (b) Combined results of nine females from a monosyllabic population of E. ephippiger (one further female who did not express a preference is not included). The non-native song was a four-syllabic chirp of polysyllabic E. ephippiger song played in alternation with one to six syllabic chirps of native song. The figures show curves fitted to the cubic spline data points (solid line) and ±1 SE from 1000 bootstrapped replicates (dashed lines). Open circles are the proportion of choices of the native song type shown by the females. The superimposed histograms show the distribution of the male trait.
Fig. 3 shows the preferences of females from a polysyllabic population of E. ephippiger in comparison with the distribution of the trait in males from the same population. Although nearly matching, the most preferred song type is five syllables, which is at a low frequency in the population. This will be typical of most directional preferences—they are directional only over a range which determines the “recognized” region. This result could therefore also be described as broadly stabilizing, indicating the limited usefulness of these widely used descriptive terms for preference shape. There is a general similarity between the shape of the preference and the distribution of the male trait in all the populations studied (Figs. 2 and 3).
Figure 3.
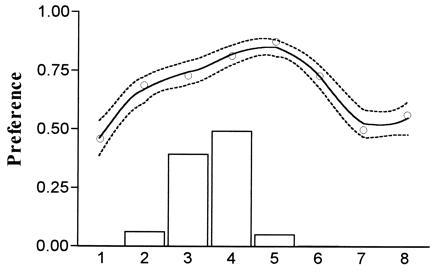
Combined results of six females from a polysyllabic population of E. ephippiger. The procedure was as before, except the non-native song type was monosyllabic, and the native was 1 to 8 syllables of polysyllabic song. The male trait distribution (histogram) is derived from over 3000 syllables from 78 males reared and recorded in the laboratory (a similar distribution is seen in the field).
Fig. 4 shows the preferences of reciprocal hybrid females from crosses between the song races. The preferences of hybrids are peaked around intermediate syllable numbers. One reciprocal (poly female × mono male) actually has an overall preference for the alternative, non-native song (in this case parental monosyllabic song) but chooses randomly when the alternative is native hybrid song of intermediate syllable number, resulting in a peak around intermediate syllable number. The peak preferences of the reciprocal genotypes are displaced toward that of the maternal genotype. The mean syllable numbers of reciprocal hybrid males from this cross are displaced in the same direction (Table 1).
Figure 4.
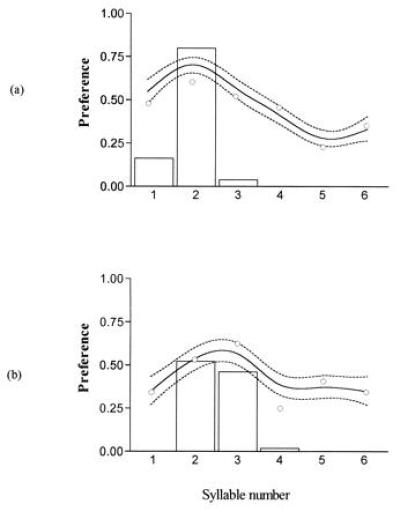
Combined results of females resulting from a cross between monosyllabic females and polysyllabic males (n = 6) (a) and polysyllabic females and monosyllabic males (n = 4) (b) of E. ephippiger. The procedure was as before, except the non-native song type was that of the monosyllabic parental population, and the native was one to six syllables of hybrid song. The male trait distributions are from the data described in Table 1.
Table 1.
Mean syllable number in the cross between the song races
| Genotype | Monosyllabic | Mono × poly | Poly × mono | Polysyllabic |
|---|---|---|---|---|
| Mean syllable number | 1 | 1.94 | 2.53 | 3.60 |
| Standard deviation | — | 0.02 | 0.02 | 0.03 |
Monosyllabic populations are monomorphic. The standard deviation estimates for the polysyllabic and hybrid genotypes are from a generalized linear model analysis. For the hybrids, the sample sizes are 840 chirps from 5 males of each genotype, and there are significant differences both among males within genotypes (F8,830 = 113, P < 0.001) and between genotypes (F1,830 = 577, P < 0.001 or F1,8 = 77, P = 0.04 if the variance among males is used as the denominator mean square). The polysyllabic analysis involved around 1500 chirps from four males each of five families, and the estimate is for the variance of family means. There were significant differences among males within families (F15,1478 = 85, P < 0.001; average SD within males = 0.06). Families did not differ if the variance among males was used as the denominator mean square (F4,15 = 1.2, not significant).
DISCUSSION
The coevolutionary model of signal-preference evolution suggests that complementarity in the two traits will be seen most of the time during evolution, with coordinated change being either maintained during the evolution of new signaling systems or achieved rapidly thereafter (24). This is perhaps the most likely evolutionary process where strong stabilizing selection predominates communication. Recently “sensory exploitation” has been proposed as an alternative model of signal-preference evolution (13). This supposes that female preference functions favor particular signals and the male trait has to evolve in response, with the driving force in evolution being the appearance of new preferences that occur independently of change in the male trait. Although one may expect the male trait to evolve rapidly to match the female preference, the theory originated with observations of substantial mismatches in signals and preferences, with males of some species lacking traits that females prefer (3, 13). For example, Xiphophorus fish have been shown to have nonmatching traits and preference functions, with females of platyfish species (lacking an exaggerated tail) preferring males with the exaggerated tails of swordtail fish (25). Basolo considers this an example of sensory exploitation, with the preference predating the evolution of the exaggerated tail (14), although others have concluded from phylogenetic information that another possibility is secondary loss of the sword in platyfish (26, 27). There is also a lack of coordination between trait and preference in the Túngara frog. Ryan and Rand (28), using playback experiments with contemporary and reconstructed ancestral calls, concluded that preference shape could be substantially out of step with the distribution of male traits during evolution. Female preference functions were wider than the current distribution of male traits, overlapping with ancestral male trait values.
The present study has allowed detailed examination of the shape of female mating preferences for an important acoustic mating signal in a bushcricket. There is general agreement between the distribution of the male trait and the female preference function in two races of one species and in a closely related congeneric species. There is no evidence of a substantial mismatch, and the races of E. ephippiger are almost certainly recently evolved (18). It therefore seems most likely that this is an example of a coevolving communication system. However, one cannot rule out the possibility that female preferences changed first, out of step with the system, invoking subsequent evolution of the male signal. Thus these data do not necessarily reject a sensory exploitation hypothesis.
Slight mismatches in trait and preference were found here in that the most preferred song type was at a low frequency in the polysyllabic population and females of strictly monomorphic monosyllabic populations would respond to bisyllabic males. We do not yet know which of the song races of E. ephippiger is ancestral; therefore, we cannot infer if the broader preferences of the monosyllabic females compared with those of the monomorphic monosyllabic congeneric species (E. terrestris) either facilitated the change to a polysyllabic communication system or results from a more recent evolution of monosyllabic song. Plainly, these mismatches are much less than those found in the frog or fish examples described above, in which females actually prefer elements of a heterospecific signal. Sensory exploitation is presumably more likely to be found in cases where the perceptive apparatus influencing the preference is under strong selection from another source (e.g., food detection or predator avoidance), producing biases in trait perception (29). Coevolution will be more likely with genuinely arbitrary preferences, independent of direct selection from other sources.
The approach to measuring female preference functions described here has the potential to greatly improve our understanding of the nature of female mating preferences (3) and is worth applying to other model systems. Few similar studies are available. Wagner et al. (12) found that females of the cricket Gryllus integer have repeatable differences in the strength of preference for different song traits (although their analysis assumed the preference functions were directional). Houde has shown that the female preference function of guppies for orange tail coloration in males varies between two populations; one population shows a strong, possibly stabilizing, pattern (30), whereas the other consists of nonchoosy females, resulting in a flat preference function (31). In an elegant series of experiments Gilburn and Day (32, 33) have used cubic spline analysis to examine female preferences of the dipteran Coelopa frigida by analyzing acceptance rates during pairing in the laboratory versus male size, for individuals of different genotypes. The preference function differs both between populations and between females of different genotypes (32). It can also differ for environmental reasons, and was unstable in the laboratory (33).
The preference functions described here are clearly genetic in origin as F1 hybrids show functions peaked at intermediate syllable numbers. The corresponding difference between reciprocal genotypes is intriguing. These findings resemble patterns found in hybrids between Teleogryllus species (34), for which there were substantial differences in the structure of male song between reciprocals, and females preferred song of the appropriate genotype (i.e., a female resulting from the cross T. oceanicus × T. commodus would prefer the song of F1 males over those of T. commodus × T. oceanicus). These results have been interpreted as evidence for a common genetic basis to signal and preference, but there are a number of alternative interpretations, including maternal effects for female preference and either similar maternal effects or sex-linkage for male song (35). Some authors have suggested sex-linked genes may play a disproportionate role in behavioral reproductive isolation (36, 37), although supporting evidence is unclear (38). Ephippiger has 15 pairs of chromosomes, so significant sex linkage here would be interesting. More studies of the shape of female preference, its genetic control, and, in particular, variability of preference shape between females are needed to assess the numerous evolutionary models of signaling, sexual selection, and speciation.
Acknowledgments
Dolph Schluter wrote the program used for the cubic spline analysis. Henry Rae and Hilary Ritchie gave invaluable help with the bushcrickets, and Andy Snedden, Iain Couzin, Jenny Gleason, and Mark Kirkpatrick provided useful discussion and comments. I am grateful to the Natural Environment Research Council (Swindon) for funding (Research Fellowship GT5/92/TLS/16).
References
- 1.Andersson M. Sexual Selection. Princeton: Princeton Univ. Press; 1994. [Google Scholar]
- 2.Gerhardt H C. Annu Rev Ecol Syst. 1994;25:293–324. [Google Scholar]
- 3.Ryan M J, Rand A S. Evolution. 1993;47:647–657. doi: 10.1111/j.1558-5646.1993.tb02118.x. [DOI] [PubMed] [Google Scholar]
- 4.Hoikkala A, Aspi J. Evolution. 1993;47:768–777. doi: 10.1111/j.1558-5646.1993.tb01232.x. [DOI] [PubMed] [Google Scholar]
- 5.Pomiankowski A, Møller A P. Proc R Soc London Ser B. 1995;260:21–29. [Google Scholar]
- 6.O’Donald P O. Genetic Models of Sexual Selection. Cambridge, U.K.: Cambridge Univ. Press; 1980. [Google Scholar]
- 7.Ewing A W, Miyan J A. Anim Behav. 1986;34:421–429. [Google Scholar]
- 8.Maynard-Smith J. Trends Ecol Evol. 1991;6:146–151. doi: 10.1016/0169-5347(91)90055-3. [DOI] [PubMed] [Google Scholar]
- 9.Paterson H E H. Evolution and the Recognition Concept of Species. Baltimore: Johns Hopkins Univ. Press; 1993. [Google Scholar]
- 10.von Helversen O, von Helversen D. In: Neural Basis of Behavioral Adaptations. Schildberger K, Elsner N, editors. Stuttgart: Fischer; 1994. pp. 253–284. [Google Scholar]
- 11.Lande R. Proc Natl Acad Sci USA. 1981;78:3721–3725. doi: 10.1073/pnas.78.6.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner W E, Jr, Murray A-M, Cade W H. Anim Behav. 1995;49:1269–1281. [Google Scholar]
- 13.Ryan M J. Oxford Surv Evol Biol. 1990;7:157–195. [Google Scholar]
- 14.Basolo A L. Proc R Soc London Ser B. 1995;259:307–311. doi: 10.1098/rspb.1995.0045. [DOI] [PubMed] [Google Scholar]
- 15.Busnel R-G, Dumortier B, Busnel M-C. Bull Biol Fr Belg. 1956;15:219–286. [Google Scholar]
- 16.Duijm M. Neth J Zool. 1990;40:428–435. [Google Scholar]
- 17.Ritchie M G. Anim Behav. 1991;42:518–520. [Google Scholar]
- 18.Ritchie M G. Anim Behav. 1992;43:845–855. [Google Scholar]
- 19.Falconer D S, Mackay T F C. Introduction to Quantitative Genetics. Harlow, U.K.: Longman; 1996. [Google Scholar]
- 20.Ritchie M G. Behav Genet. 1992;22:369–379. doi: 10.1007/BF01066668. [DOI] [PubMed] [Google Scholar]
- 21.Ritchie M G, Couzin I D, Snedden W A. Proc R Soc London Ser B. 1995;262:21–27. [Google Scholar]
- 22.Schluter D. Evolution. 1988;42:849–861. doi: 10.1111/j.1558-5646.1988.tb02507.x. [DOI] [PubMed] [Google Scholar]
- 23.Oudman L, Duijm M, Landman W. Neth J Zool. 1990;40:454–483. [Google Scholar]
- 24.Alexander R D. Evolution. 1962;16:433–467. [Google Scholar]
- 25.Basolo A L. Science. 1990;250:808–810. doi: 10.1126/science.250.4982.808. [DOI] [PubMed] [Google Scholar]
- 26.Meyer A, Morrisey J M, Schartl M. Nature (London) 1994;368:539–542. doi: 10.1038/368539a0. [DOI] [PubMed] [Google Scholar]
- 27.Pomiankowski A. Nature (London) 1994;368:494–495. doi: 10.1038/368494a0. [DOI] [PubMed] [Google Scholar]
- 28.Ryan M J, Rand A S. Science. 1995;269:390–392. doi: 10.1126/science.269.5222.390. [DOI] [PubMed] [Google Scholar]
- 29.Endler J A. Am Nat. 1992;139:S125–S153. [Google Scholar]
- 30.Houde A E. Evolution. 1987;41:1–10. doi: 10.1111/j.1558-5646.1987.tb05766.x. [DOI] [PubMed] [Google Scholar]
- 31.Houde A E. Anim Behav. 1988;36:510–516. [Google Scholar]
- 32.Gilburn A S, Day T H. Proc R Soc London Ser B. 1994;257:303–309. [Google Scholar]
- 33.Gilburn A S, Day T H. Genet Res. 1994;64:19–25. [Google Scholar]
- 34.Hoy R R, Hahn J, Paul R C. Science. 1977;195:82–84. doi: 10.1126/science.831260. [DOI] [PubMed] [Google Scholar]
- 35.Butlin R K, Ritchie M G. Biol J Linn Soc. 1989;37:237–246. [Google Scholar]
- 36.Ewing A W. Anim Behav. 1969;17:555–560. doi: 10.1016/0003-3472(69)90164-x. [DOI] [PubMed] [Google Scholar]
- 37.Reinhold K. Biol J Linn Soc. 1994;52:305–316. [Google Scholar]
- 38.Charlesworth B, Coyne J A, Barton N H. Am Nat. 1987;130:113–146. [Google Scholar]


