Abstract
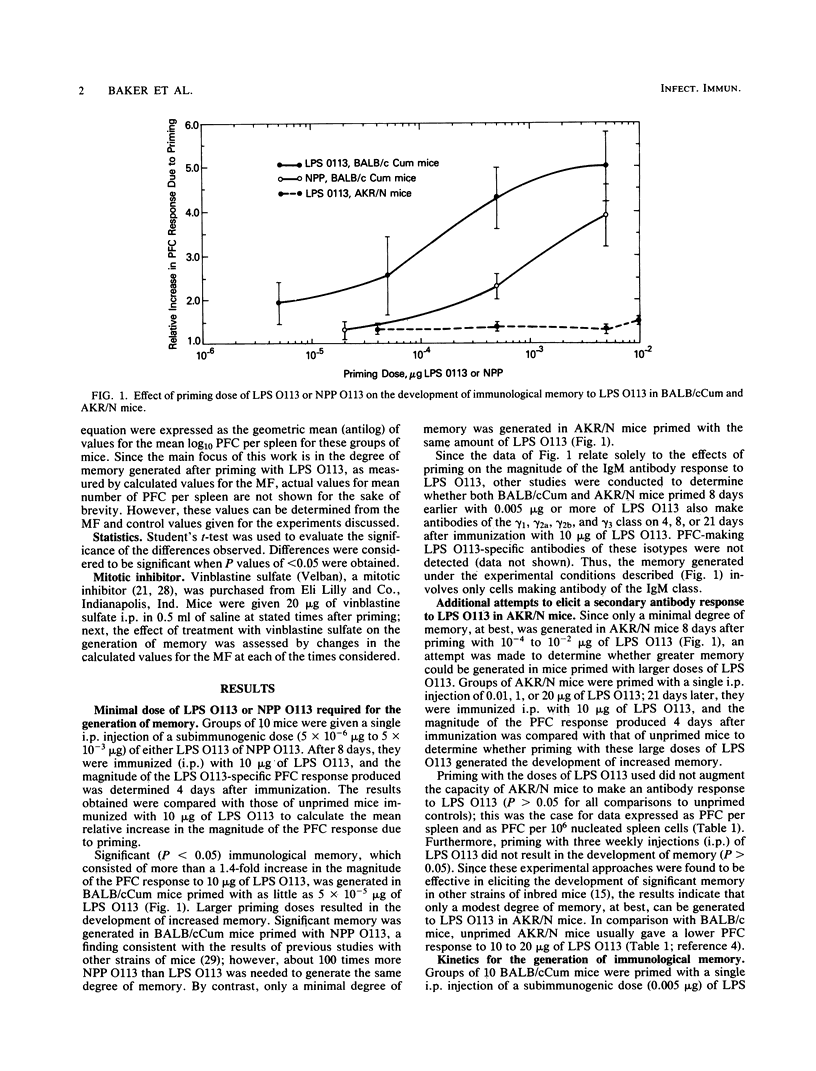
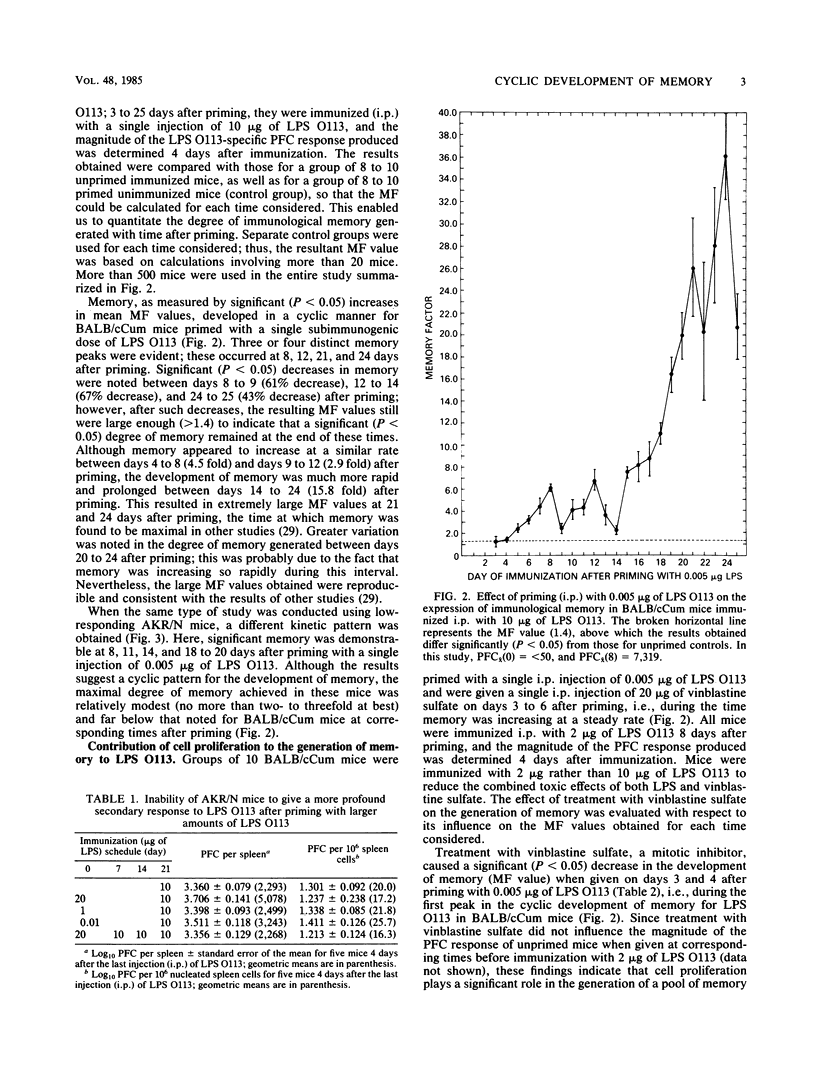
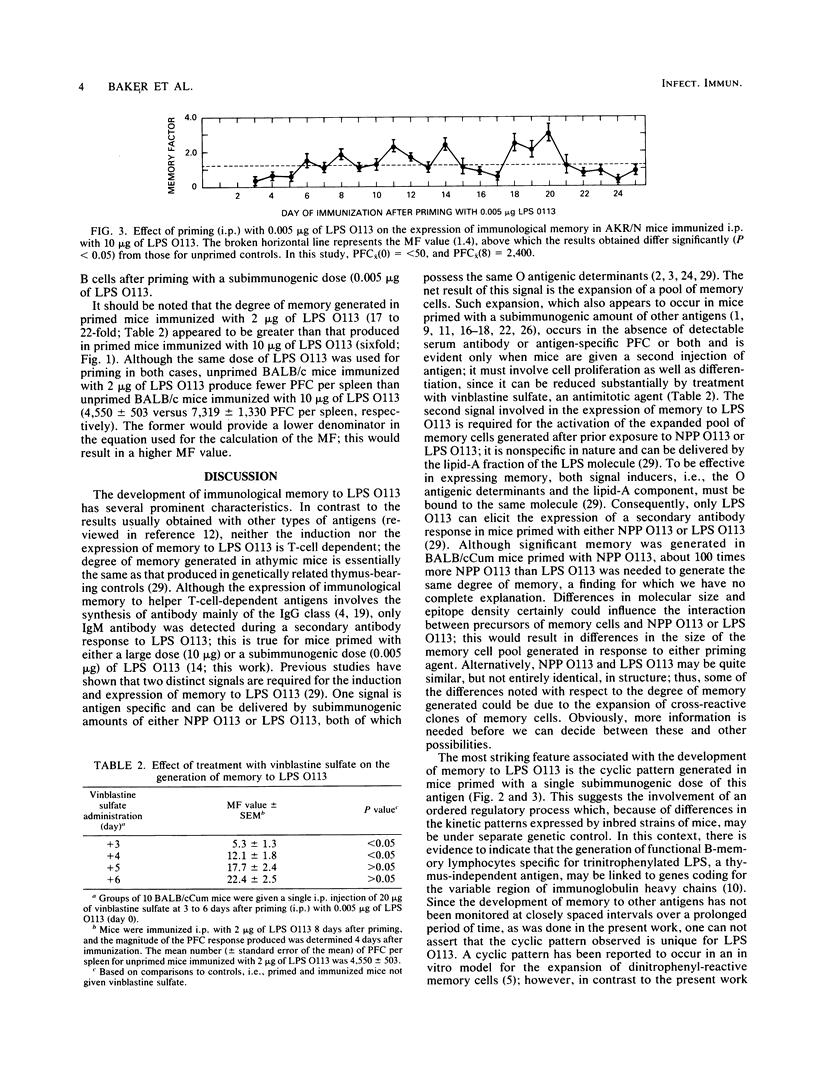
Immunological memory to the lipopolysaccharide of Escherichia coli O113 was generated in strains of inbred mice given a single subimmunogenic dose of either E. coli O113 lipopolysaccharide or the native protoplasmic polysaccharide of E. coli O113. Such memory, which only involved antibody of the immunoglobulin M class, developed in a cyclic manner that was characteristic for the strain of mice used. It involved cell proliferation as well as differentiation and persisted for at least 25 days after priming with a single injection of a subimmunogenic dose of E. coli O113 lipopolysaccharide.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANACKER R. L., FINKELSTEIN R. A., HASKINS W. T., LANDY M., MILNER K. C., RIBI E., STASHAK P. W. ORIGIN AND PROPERTIES OF NATURALLY OCCURRING HAPTEN FROM ESCHERICHIA COLI. J Bacteriol. 1964 Dec;88:1705–1720. doi: 10.1128/jb.88.6.1705-1720.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker R. L., Bickel W. D., Haskins W. T., Milner K. C., Ribi E., Rudbach J. A. Frequency of occurrence of native hapten among enterobacterial species. J Bacteriol. 1966 Apr;91(4):1427–1433. doi: 10.1128/jb.91.4.1427-1433.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braley-Mullen H. Antigen requirements for induction of B-memory cells: studies with dinitrophenyl coupled to T-dependent and T-independent carriers. J Exp Med. 1978 Jun 1;147(6):1824–1831. doi: 10.1084/jem.147.6.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks K. H., Feldbush T. L. Generation of antibody-mediated regulation during in vitro clonal expansion of memory B lymphocytes. J Immunol. 1981 Sep;127(3):963–967. [PubMed] [Google Scholar]
- Brooks K. H., Feldbush T. L. In vitro antigen-mediated clonal expansion of memory B lymphocytes. J Immunol. 1981 Sep;127(3):959–963. [PubMed] [Google Scholar]
- Brooks K. H., Feldbush T. L., van der Hoven A. The generation of memory B-cell subpopulations capable of proliferation and expansion of the pool: effect of time and antigen. Cell Immunol. 1980 Mar 15;50(2):392–404. doi: 10.1016/0008-8749(80)90293-2. [DOI] [PubMed] [Google Scholar]
- Celada F. The cellular basis of immunologic memory. Prog Allergy. 1971;15:223–267. [PubMed] [Google Scholar]
- Chiller J. M., Weigle W. O. Termination of tolerance to human gamma globulin in mice by antigen and bacterial lipopolysaccharide (endotoxin). J Exp Med. 1973 Mar 1;137(3):740–750. doi: 10.1084/jem.137.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colle J. H., Motta I., Shidani B., Truffa-Bachi P. Igh-V or closely linked gene(s) control immunological memory to a thymus-independent antigen. Nature. 1983 Feb 3;301(5899):428–429. doi: 10.1038/301428a0. [DOI] [PubMed] [Google Scholar]
- Dresser D. W., Popham A. M. The influence of T cells on the initiation and expression of immunological memory. Immunology. 1979 Oct;38(2):265–274. [PMC free article] [PubMed] [Google Scholar]
- Gottlieb C. F. Application of transformations to normalize the distribution of plaque-forming cells. J Immunol. 1974 Jul;113(1):51–57. [PubMed] [Google Scholar]
- Hiernaux J. R., Baker P. J., Delisi C., Rudbach J. A. Modulation of the immune response to lipopolysaccharide. J Immunol. 1982 Mar;128(3):1054–1058. [PubMed] [Google Scholar]
- Hiernaux J. R., Jones J. M., Rudbach J. A., Rollwagen F., Baker P. J. Antibody response of immunodeficient (xid) CBA/N mice to Escherichia coli 0113 lipopolysaccharide, a thymus-independent antigen. J Exp Med. 1983 Apr 1;157(4):1197–1207. doi: 10.1084/jem.157.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa T. Studies on B-cell memory. II. T-cell independent antigen can induce B-cell memory. Immunology. 1979 Oct;38(2):291–299. [PMC free article] [PubMed] [Google Scholar]
- Hünig T., Schimpl A., Wecker E. Autoradiographic studies on the proliferation of antibody-producing cells in vitro. J Exp Med. 1974 Mar 1;139(3):754–760. doi: 10.1084/jem.139.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macario A. J., Conway de Macario E. Silent generation of memory in genetic low-responder mice after a single contract with a bacterial antigen. Cell Immunol. 1978 Apr;37(1):263–267. doi: 10.1016/0008-8749(78)90193-4. [DOI] [PubMed] [Google Scholar]
- Mitchison N. A. The carrier effect in the secondary response to hapten-protein conjugates. I. Measurement of the effect with transferred cells and objections to the local environment hypothesis. Eur J Immunol. 1971 Jan;1(1):10–17. doi: 10.1002/eji.1830010103. [DOI] [PubMed] [Google Scholar]
- Nossal G. J., Austin C. M., Ada G. L. Antigens in immunity. VII. Analysis of immunological memory. Immunology. 1965 Oct;9(4):333–348. [PMC free article] [PubMed] [Google Scholar]
- Perkins E. H., Sado T., Makinodan T. Recruitment and proliferation of immunocompetent cells during the log phase of the primary antibody response. J Immunol. 1969 Oct;103(4):668–678. [PubMed] [Google Scholar]
- Roelants G. E., Askonas B. A. Immunological B memory in thymus deprived mice. Nat New Biol. 1972 Sep 13;239(89):63–64. doi: 10.1038/newbio239063a0. [DOI] [PubMed] [Google Scholar]
- Rudbach J. A., Akiya F. I., Elin R. J., Hochstein H. D., Luoma M. K., Milner E. C., Milner K. C., Thomas K. R. Preparation and properties of a national reference endotoxin. J Clin Microbiol. 1976 Jan;3(1):21–25. doi: 10.1128/jcm.3.1.21-25.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudbach J. A., Reed N. D. Immunological responses of mice to lipopolysaccharide: lack of secondary responsiveness by C3H/HeJ mice. Infect Immun. 1977 May;16(2):513–517. doi: 10.1128/iai.16.2.513-517.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott C. F., Merchant B. Carrier-specific immune memory to a thymus-independent antigen in congenitally athymic mice. J Immunol. 1979 May;122(5):1710–1718. [PubMed] [Google Scholar]
- Schrader J. W. The role of T cells in IgG production; thymus-dependent antigens induce B cell memory in the absence of T cells. J Immunol. 1975 Jun;114(6):1665–1669. [PubMed] [Google Scholar]
- Syeklocha D., Siminovitch L., Till J. E., McCulloch E. A. The proliferative state of antigen-sensitive precursors of hemolysin-producing cells, determined by the use of the inhibitor, vinblastine. J Immunol. 1966 Mar;96(3):472–477. [PubMed] [Google Scholar]
- Von Eschen K. B., Rudbach J. A. Immunological responses of mice to native protoplasmic polysaccharide and lipopolysaccharide: functional separation of the two signals required to stimulate a secondary antibody response. J Exp Med. 1974 Dec 1;140(6):1604–1614. doi: 10.1084/jem.140.6.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]


