Abstract
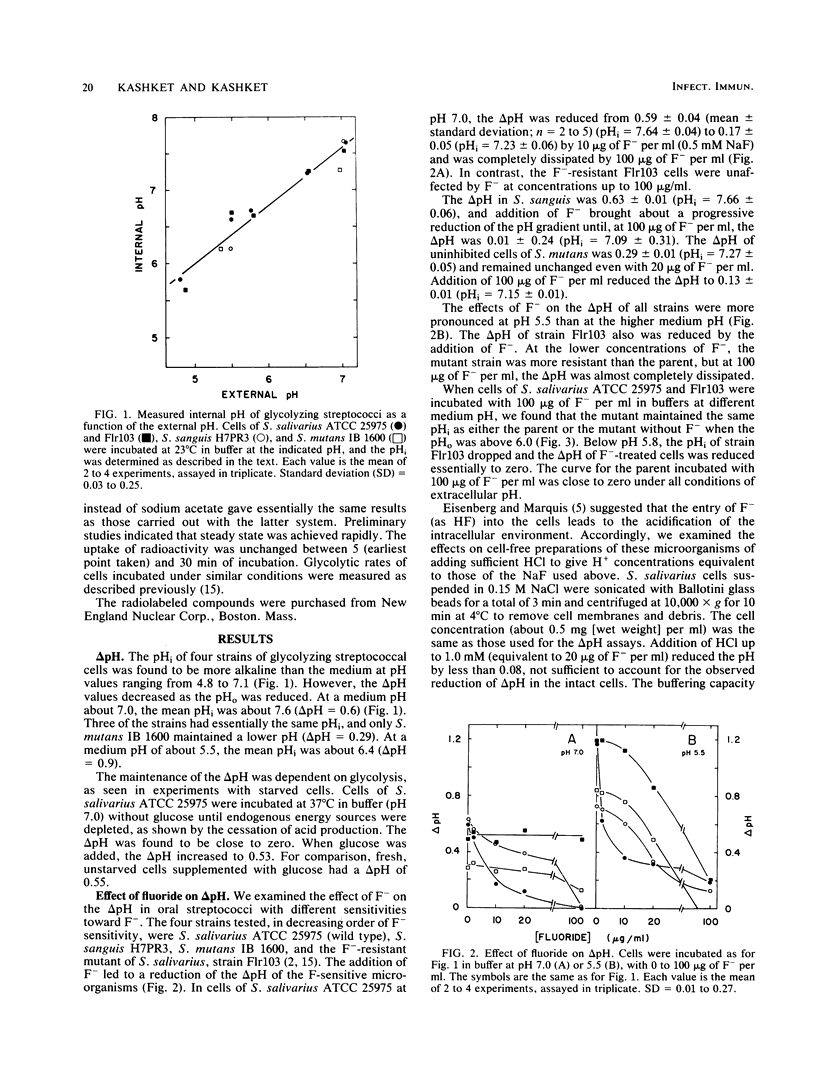
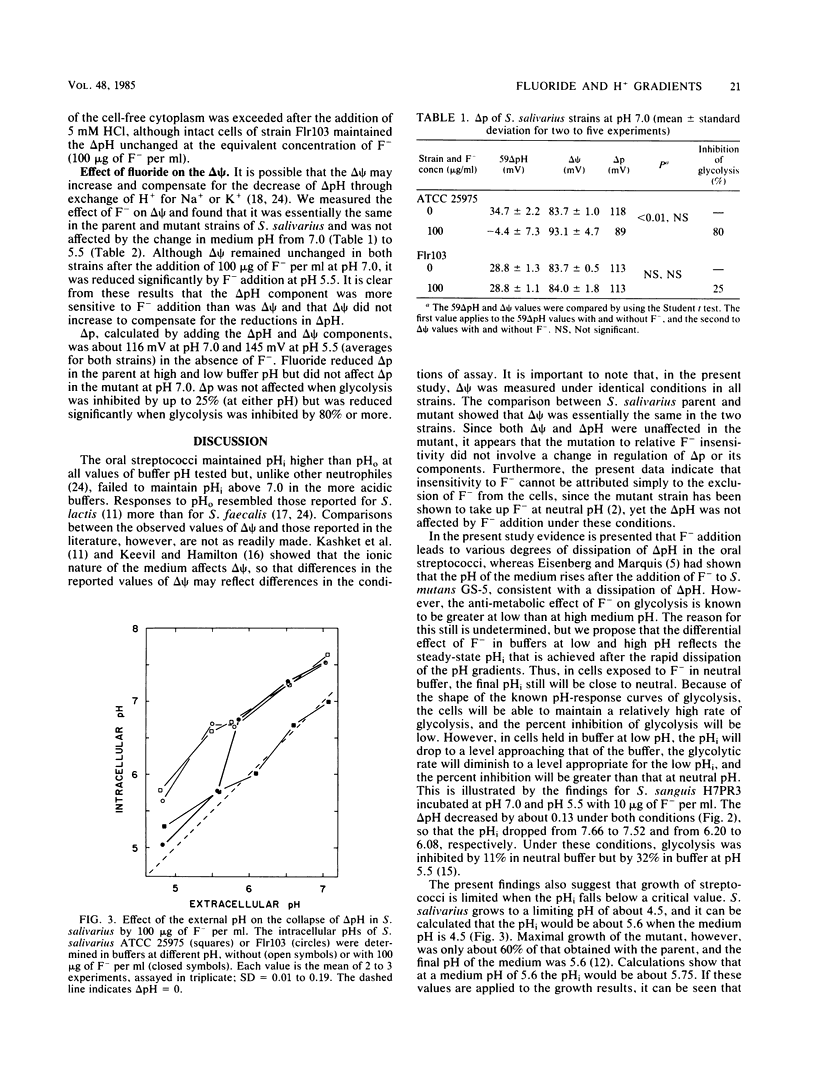
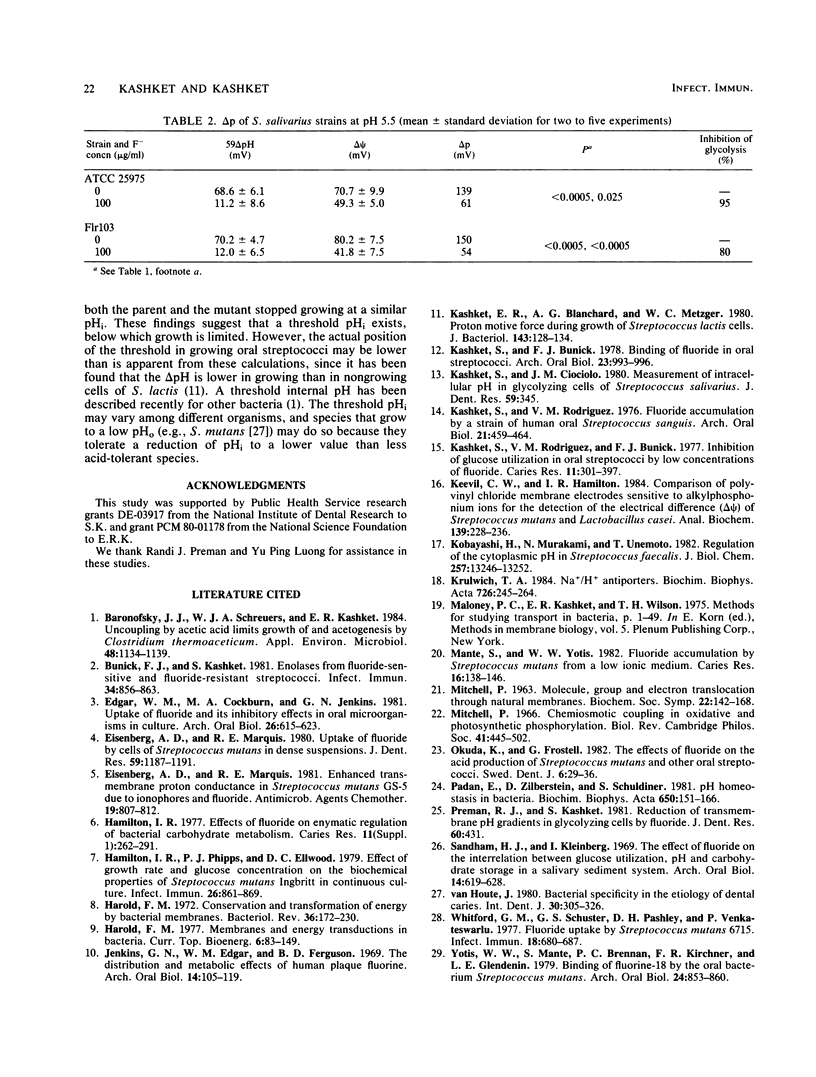
Strains of oral streptococci maintain an intracellular pH (pHi) that is more alkaline than the external pH (pHo). The delta pH (pHi-pHo) was about 0.6 in neutral media and about 0.9 in media of pH 5.5. Addition of 10 micrograms of F- per ml at pH 7.0 reduced the delta pH in Streptococcus salivarius to 0.17. The reduction of delta pH in S. sanguis H7PR3 was less pronounced, whereas the delta pH in S. mutans IB 1600 was unaffected. The F- -resistant mutant of S. salivarius, strain Flr103, maintained a delta pH of 0.51 with 100 micrograms of F- per ml. Addition of F- to cells in media below pH 6.0 led to a reduction of delta pH in all strains. The anion had no effect on the transmembrane electrical gradient of either mutant or parental cells of S. salivarius at pH 7.0. The principal effect of F- addition at neutral pH, therefore, was on the delta pH component of the proton motive force. At pH 5.5, 100 micrograms of F- per ml reduced the transmembrane electrical gradient from 71 to 40 mV in the parent and from 80 to 42 mV in the mutant. We propose that the greater sensitivity of cells to F- at lower medium pH stems from the rapid dissipation of delta pH by the anion. Thus, pH equilibration in media of low pH would lead to a greater reduction of metabolic activity than when it occurs in media at neutral pH. The data also suggest that the growth of streptococci, with or without added F-, is limited when the intracellular pH falls below about 5.7.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baronofsky J. J., Schreurs W. J., Kashket E. R. Uncoupling by Acetic Acid Limits Growth of and Acetogenesis by Clostridium thermoaceticum. Appl Environ Microbiol. 1984 Dec;48(6):1134–1139. doi: 10.1128/aem.48.6.1134-1139.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunick F. J., Kashket S. Enolases from fluoride-sensitive and fluoride-resistant streptococci. Infect Immun. 1981 Dec;34(3):856–863. doi: 10.1128/iai.34.3.856-863.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar W. M., Cockburn M. A., Jenkins G. N. Uptake of fluoride and its inhibitory effects in oral microorganisms in culture. Arch Oral Biol. 1981;26(7):615–623. doi: 10.1016/0003-9969(81)90024-8. [DOI] [PubMed] [Google Scholar]
- Eisenberg A. D., Marquis R. E. Enhanced transmembrane proton conductance in Streptococcus mutans GS-5 due to ionophores and fluoride. Antimicrob Agents Chemother. 1981 May;19(5):807–812. doi: 10.1128/aac.19.5.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg A. D., Marquis R. E. Uptake of fluoride by cells of Streptococcus mutans in dense suspensions. J Dent Res. 1980 Jul;59(7):1187–1191. doi: 10.1177/00220345800590072801. [DOI] [PubMed] [Google Scholar]
- Hamilton I. R. Effects of fluoride on enzymatic regulation of bacterial carbohydrate metabolism. Caries Res. 1977;11 (Suppl 1):262–291. doi: 10.1159/000260304. [DOI] [PubMed] [Google Scholar]
- Hamilton I. R., Phipps P. J., Ellwood D. C. Effect of growth rate and glucose concentration on the biochemical properties of Streptococcus mutans Ingbritt in continuous culture. Infect Immun. 1979 Dec;26(3):861–869. doi: 10.1128/iai.26.3.861-869.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins G. N., Edgar W. M. The distribution and metabolic effects of human plaque fluorine. Arch Oral Biol. 1969 Jan;14(1):105–119. doi: 10.1016/0003-9969(69)90025-9. [DOI] [PubMed] [Google Scholar]
- Kashket E. R., Blanchard A. G., Metzger W. C. Proton motive force during growth of Streptococcus lactis cells. J Bacteriol. 1980 Jul;143(1):128–134. doi: 10.1128/jb.143.1.128-134.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket S., Bunick F. J. Binding of fluoride in oral streptococci. Arch Oral Biol. 1978;23(11):993–996. doi: 10.1016/0003-9969(78)90255-8. [DOI] [PubMed] [Google Scholar]
- Kashket S., Rodriguez V. M., Bunick F. J. Inhibition of glucose utilization in oral streptococci by low concentrations of fluoride. Caries Res. 1977;11(6):301–307. doi: 10.1159/000260283. [DOI] [PubMed] [Google Scholar]
- Kashket S., Rodriguez V. M. Fluoride accumulation by a strain of human oral Streptococcus sanguis. Arch Oral Biol. 1976;21(8):459–464. doi: 10.1016/0003-9969(76)90103-5. [DOI] [PubMed] [Google Scholar]
- Keevil C. W., Hamilton I. R. Comparison of polyvinyl chloride membrane electrodes sensitive to alkylphosphonium ions for the determination of the electrical difference (delta psi) of Streptococcus mutans and Lactobacillus casei. Anal Biochem. 1984 May 15;139(1):228–236. doi: 10.1016/0003-2697(84)90410-x. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Murakami N., Unemoto T. Regulation of the cytoplasmic pH in Streptococcus faecalis. J Biol Chem. 1982 Nov 25;257(22):13246–13252. [PubMed] [Google Scholar]
- Krulwich T. A. Na+/H+ antiporters. Biochim Biophys Acta. 1983 Dec 30;726(4):245–264. doi: 10.1016/0304-4173(83)90011-3. [DOI] [PubMed] [Google Scholar]
- Mante S., Yotis W. W. Fluoride accumulation by Streptococcus mutans from a low ionic medium. Caries Res. 1982;16(2):138–146. doi: 10.1159/000260590. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Okuda K., Frostell G. The effect of fluoride on the acid production of Streptococcus mutans and other oral streptococci. Swed Dent J. 1982;6(1):29–36. [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Schuldiner S. pH homeostasis in bacteria. Biochim Biophys Acta. 1981 Dec;650(2-3):151–166. doi: 10.1016/0304-4157(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Sandham H. J., Kleinberg I. The effect of fluoride on the interrelation between glucose utilization, pH and carbohydrate storage in a salivary sediment system. Arch Oral Biol. 1969 Jun;14(6):619–628. doi: 10.1016/0003-9969(69)90185-x. [DOI] [PubMed] [Google Scholar]
- Whitford G. M., Schuster G. S., Pashley D. H., Venkateswarlu P. Fluoride uptake by Streptococcus mutans 6715. Infect Immun. 1977 Dec;18(3):680–687. doi: 10.1128/iai.18.3.680-687.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotis W. W., Mante S., Brennan P. C., Kirchner F. R., Glendenin L. E. Binding of fluorine-18 by the oral bacterium, Streptococcus mutans. Arch Oral Biol. 1979;24(10-11):853–860. doi: 10.1016/0003-9969(79)90050-5. [DOI] [PubMed] [Google Scholar]
- van Houte J. Bacterial specificity in the etiology of dental caries. Int Dent J. 1980 Dec;30(4):305–326. [PubMed] [Google Scholar]


