Abstract
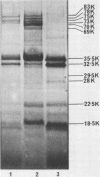
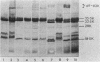
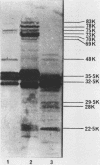
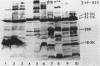
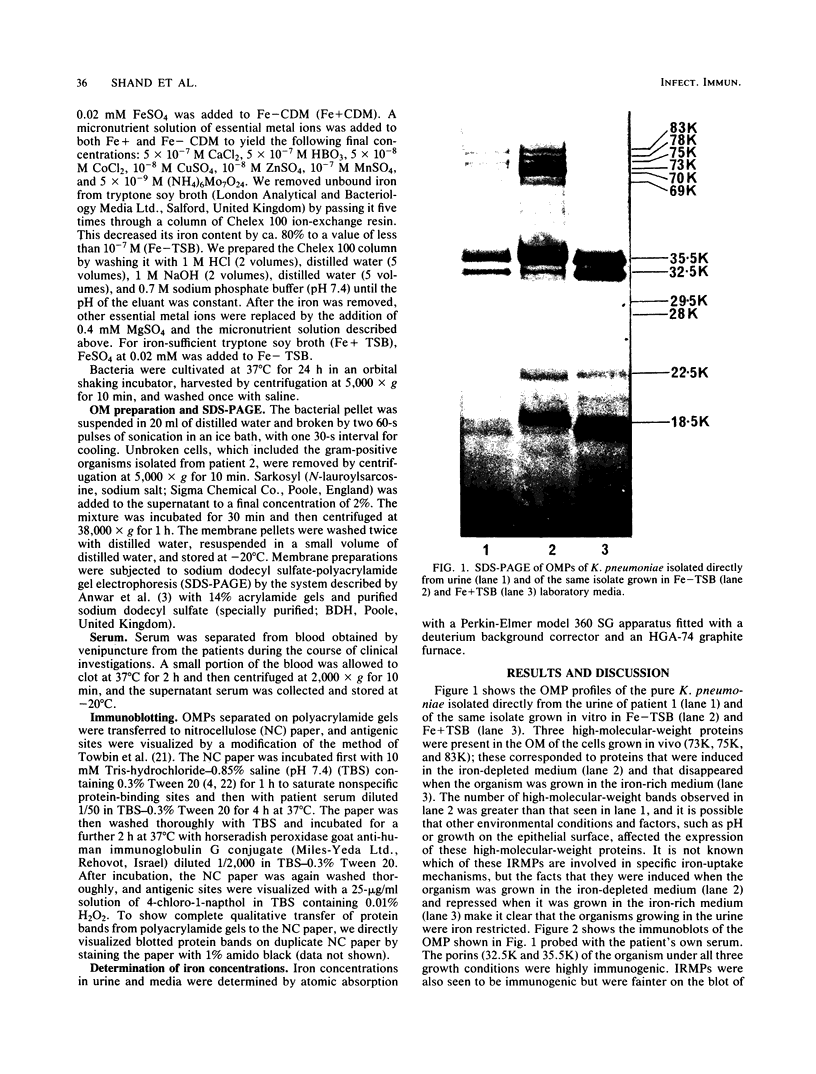
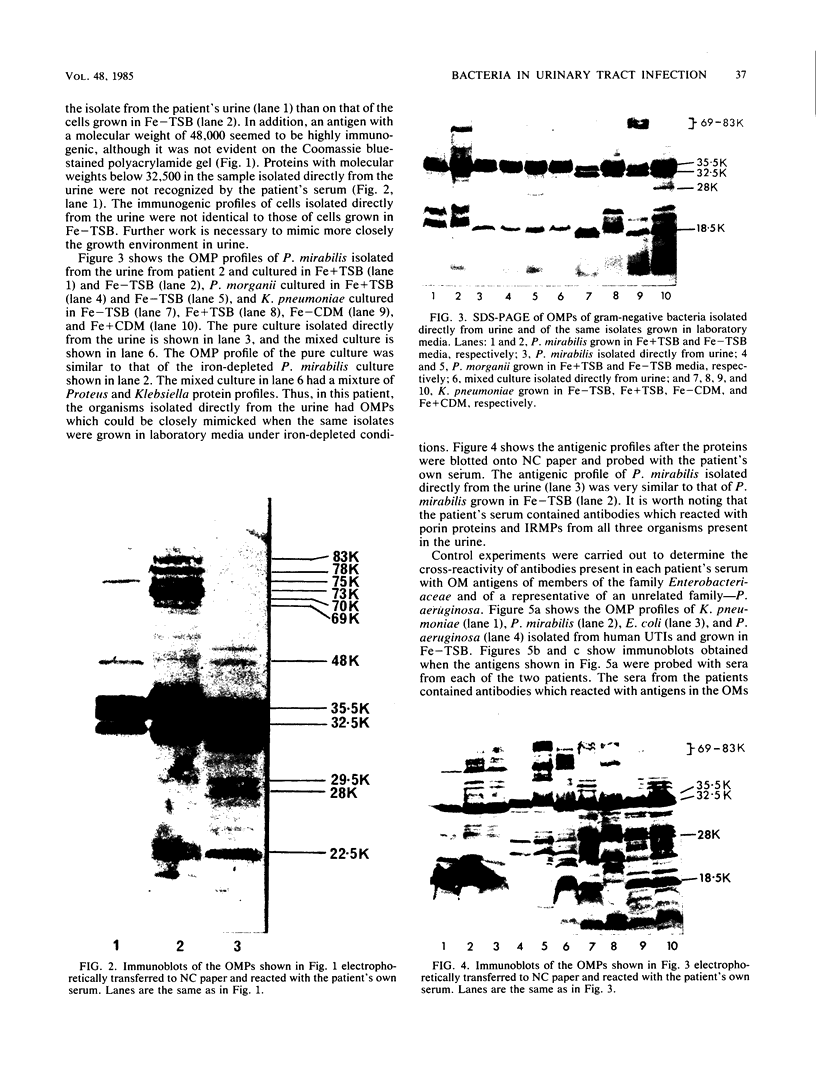
The outer membrane protein composition of bacteria isolated directly and without subculturing from the urine of two patients with urinary tract infections was investigated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The results indicated that the bacteria grew under iron-restricted conditions, as revealed by the expression of several high-molecular-weight outer membrane proteins which could also be observed when the same isolates were grown under iron-depleted conditions in laboratory media. The antigenicity of outer membrane components of the bacteria isolated was studied by immunoblotting with serum samples from the patients. The results indicated that the sera from the patients contained antibodies against major outer membrane components of the bacteria present in the urine, including the iron-regulated membrane proteins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anwar H., Brown M. R., Cozens R. M., Lambert P. A. Isolation and characterization of the outer and cytoplasmic membranes of Pseudomonas cepacia. J Gen Microbiol. 1983 Feb;129(2):499–507. doi: 10.1099/00221287-129-2-499. [DOI] [PubMed] [Google Scholar]
- Anwar H., Brown M. R., Lambert P. A. Effect of nutrient depletion on sensitivity of Pseudomonas cepacia to phagocytosis and serum bactericidal activity at different temperatures. J Gen Microbiol. 1983 Jul;129(7):2021–2027. doi: 10.1099/00221287-129-7-2021. [DOI] [PubMed] [Google Scholar]
- Anwar H., Lambert P. A., Brown M. R. Influence of sodium dodecyl sulphate quality on the electrophoretic mobility of the outer membrane proteins of mucoid and non-mucoid Pseudomonas aeruginosa. Biochim Biophys Acta. 1983 Dec 13;761(2):119–125. doi: 10.1016/0304-4165(83)90220-9. [DOI] [PubMed] [Google Scholar]
- Batteiger B., Newhall W. J., 5th, Jones R. B. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. doi: 10.1016/0022-1759(82)90089-8. [DOI] [PubMed] [Google Scholar]
- Brown M. R. Nutrient depletion and antibiotic susceptibility. J Antimicrob Chemother. 1977 May;3(3):198–201. doi: 10.1093/jac/3.3.198. [DOI] [PubMed] [Google Scholar]
- Brynger H., Brunner F. P., Chantler C., Donckerwolcke R. A., Jacobs C., Kramer P., Selwood N. H., Wing A. J. Combined report on regular dialysis and transplantation in Europe. X, 1979. Proc Eur Dial Transplant Assoc. 1980;17:2–86. [PubMed] [Google Scholar]
- Bullen J. J. The significance of iron in infection. Rev Infect Dis. 1981 Nov-Dec;3(6):1127–1138. doi: 10.1093/clinids/3.6.1127. [DOI] [PubMed] [Google Scholar]
- Finch J. E., Brown M. R. Effect of growth environment on Pseudomonas aeruginosa killing by rabbit polymorphonuclear leudocytes and cationic proteins. Infect Immun. 1978 May;20(2):340–346. doi: 10.1128/iai.20.2.340-346.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman L. R. Urinary-tract infections in the elderly. N Engl J Med. 1983 Dec 8;309(23):1451–1452. doi: 10.1056/NEJM198312083092309. [DOI] [PubMed] [Google Scholar]
- Greenwood D., O'Grady F. An in vitro model of the urinary bladder. J Antimicrob Chemother. 1978 Mar;4(2):113–120. doi: 10.1093/jac/4.2.113. [DOI] [PubMed] [Google Scholar]
- Kaye D. Urinary tract infections in the elderly. Bull N Y Acad Med. 1980 Mar;56(2):209–220. [PMC free article] [PubMed] [Google Scholar]
- Klemperer R. M., Ismail N. T., Brown M. R. Effect of R plasmid RPI on the nutritional requirements of Escherichia coli in batch culture. J Gen Microbiol. 1979 Dec;115(2):325–331. doi: 10.1099/00221287-115-2-325. [DOI] [PubMed] [Google Scholar]
- Sciortino C. V., Finkelstein R. A. Vibrio cholerae expresses iron-regulated outer membrane proteins in vivo. Infect Immun. 1983 Dec;42(3):990–996. doi: 10.1128/iai.42.3.990-996.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang V. C., Peralta J. M., Simons A. R. Enzyme-linked immunoelectrotransfer blot techniques (EITB) for studying the specificities of antigens and antibodies separated by gel electrophoresis. Methods Enzymol. 1983;92:377–391. doi: 10.1016/0076-6879(83)92032-3. [DOI] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron withholding: a defense against infection and neoplasia. Physiol Rev. 1984 Jan;64(1):65–102. doi: 10.1152/physrev.1984.64.1.65. [DOI] [PubMed] [Google Scholar]