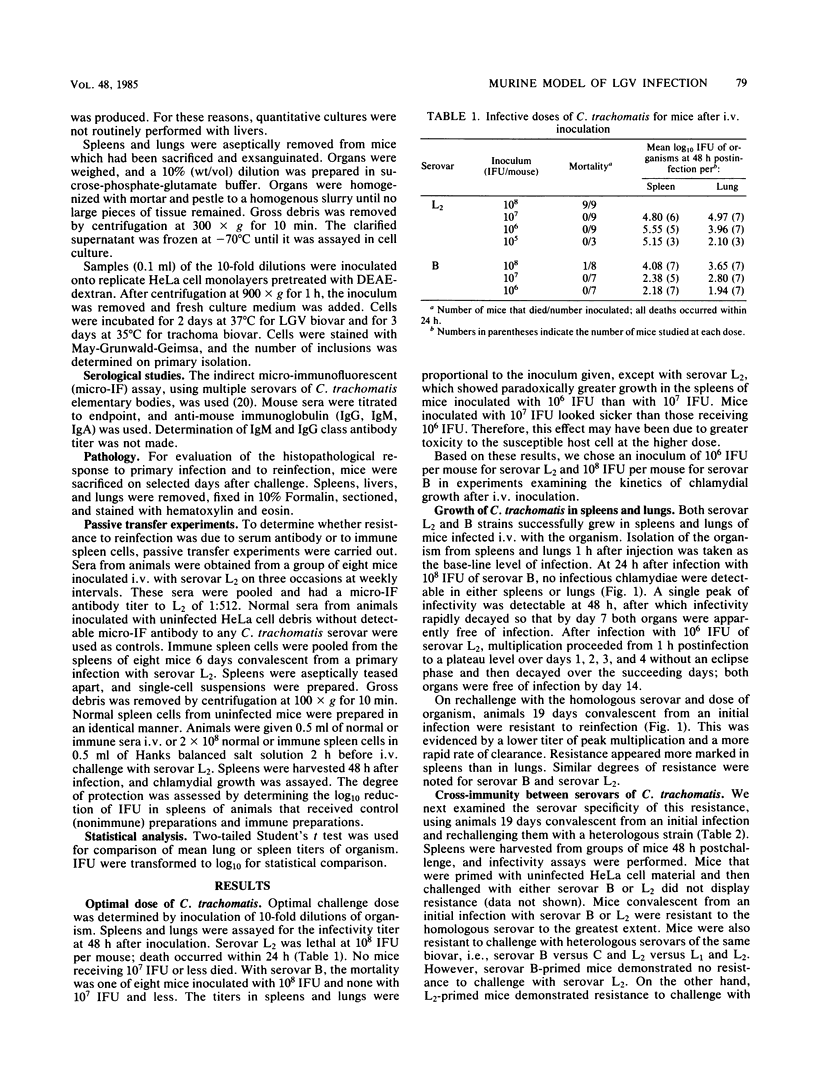
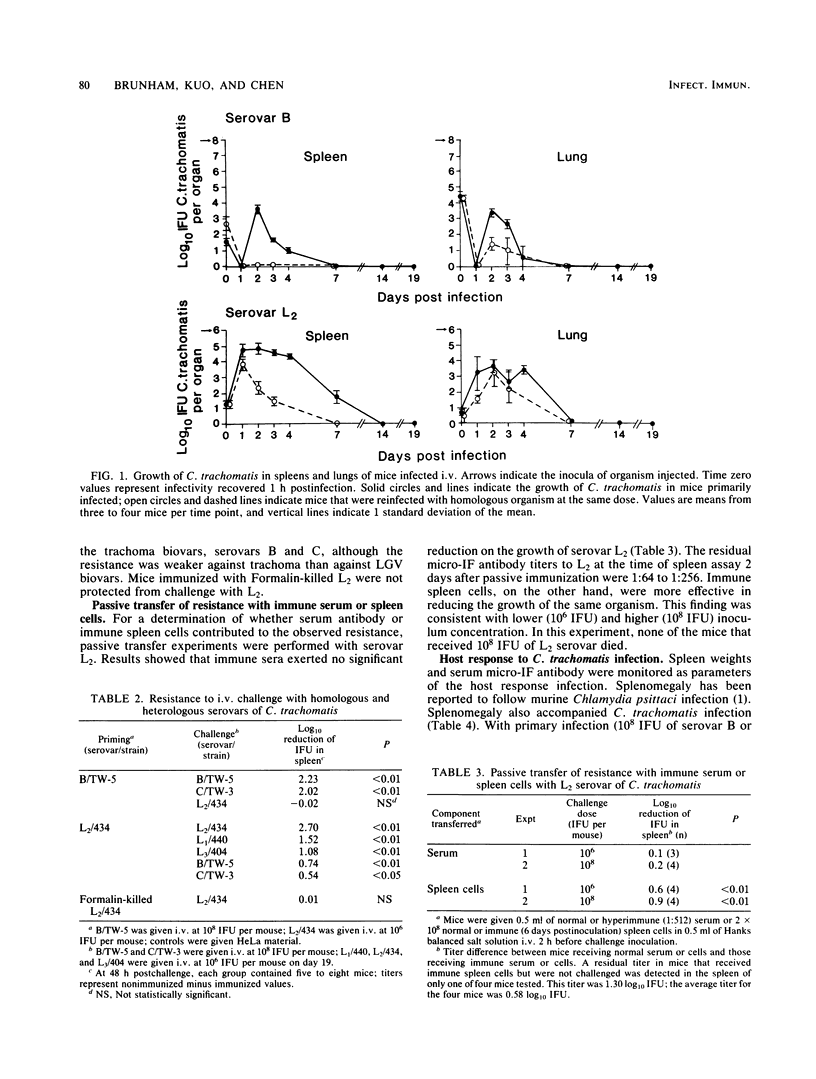
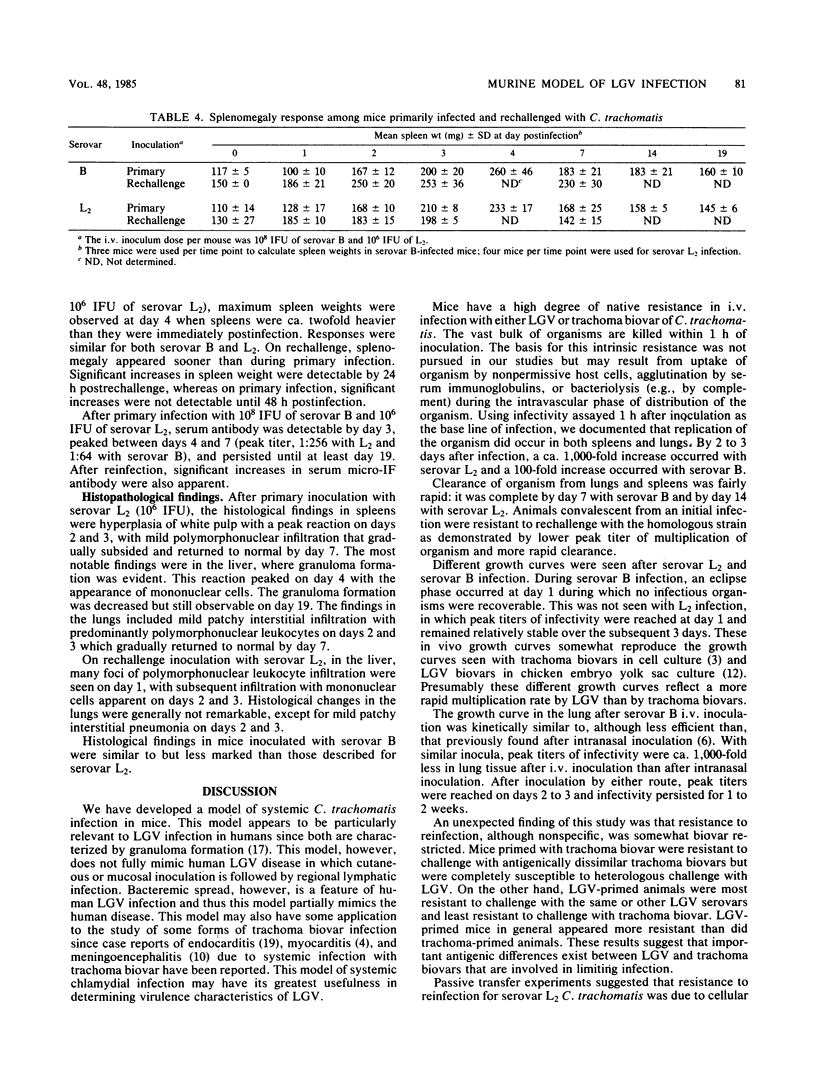
Abstract
We developed a murine model of systemic infection with Chlamydia trachomatis biovar lymphogranuloma venereum (LGV). The pathological features of this infection resemble those of human LGV infection since both are characterized by granuloma formation. Mice developed resistance to reinfection with LGV, and this resistance was based on cellular immune mechanisms since it was transferable with immune spleen cells but not with immune serum. Resistance required viable organisms for induction. We compared LGV biovar infection with trachoma biovar infection. Trachoma biovar produced similar but less marked microbiological and pathological features. Cross-immunity was less apparent between serovars from trachoma and LGV biovars than it was between serovars within the same biovar. This model of systemic C. trachomatis infection will be useful in exploring virulence features of LGV.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byrne G. I., Faubion C. L. Lymphokine-mediated microbistatic mechanisms restrict Chlamydia psittaci growth in macrophages. J Immunol. 1982 Jan;128(1):469–474. [PubMed] [Google Scholar]
- FURNESS G., FRASER E. F. One-step growth curves for inclusion blennorrhoea virus in heLa cell monolayers. J Gen Microbiol. 1962 Feb;27:299–304. doi: 10.1099/00221287-27-2-299. [DOI] [PubMed] [Google Scholar]
- Kazar J., Gillmore J. D., Gordon F. B. Effect of Interferon and Interferon Inducers on Infections with a Nonviral Intracellular Microorganism, Chlamydia trachomatis. Infect Immun. 1971 Jun;3(6):825–832. doi: 10.1128/iai.3.6.825-832.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. T., Weiss E. Lack of deoxyribonucleic acid homology between species of the genus Chlamydia. J Bacteriol. 1968 Oct;96(4):1421–1423. doi: 10.1128/jb.96.4.1421-1423.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C., Chen W. J. A mouse model of Chlamydia trachomatis pneumonitis. J Infect Dis. 1980 Feb;141(2):198–202. doi: 10.1093/infdis/141.2.198. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B. The relationship of delayed hypersensitivity to acquired cellular resistance. Br Med Bull. 1967 Jan;23(1):52–54. doi: 10.1093/oxfordjournals.bmb.a070516. [DOI] [PubMed] [Google Scholar]
- Myhre E. B., Mårdh P. A. Chlamydia trachomatis infection in a patient with meningoencephalitis. N Engl J Med. 1981 Apr 9;304(15):910–911. doi: 10.1056/NEJM198104093041514. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn E. R. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci U S A. 1984 Feb;81(3):908–912. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringel R. E., Brenner J. I., Rennels M. B., Huang S. W., Wang S. P., Grayston J. T., Berman M. A. Serologic evidence for Chlamydia trachomatis myocarditis. Pediatrics. 1982 Jul;70(1):54–56. [PubMed] [Google Scholar]
- Rothermel C. D., Byrne G. I., Havell E. A. Effect of interferon on the growth of Chlamydia trachomatis in mouse fibroblasts (L cells). Infect Immun. 1983 Jan;39(1):362–370. doi: 10.1128/iai.39.1.362-370.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter J. Chlamydial infections (third of three parts). N Engl J Med. 1978 Mar 9;298(10):540–549. doi: 10.1056/NEJM197803092981005. [DOI] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Inhibition of the growth of Rickettsia prowazekii in cultured fibroblasts by lymphokines. J Exp Med. 1983 Mar 1;157(3):974–986. doi: 10.1084/jem.157.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. P., Grayston J. T., Alexander E. R., Holmes K. K. Simplified microimmunofluorescence test with trachoma-lymphogranuloma venereum (Chlamydia trachomatis) antigens for use as a screening test for antibody. J Clin Microbiol. 1975 Mar;1(3):250–255. doi: 10.1128/jcm.1.3.250-255.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Schramek S., Wilson N. N., Newman L. W. Deoxyribonucleic Acid Heterogeneity Between Human and Murine Strains of Chlamydia trachomatis. Infect Immun. 1970 Jul;2(1):24–28. doi: 10.1128/iai.2.1.24-28.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. M., Schachter J., Drutz D. J., Sumaya C. V. Pneumonia due to Chlamydia trachomatis in the immunocompromised (nude) mouse. J Infect Dis. 1981 Feb;143(2):238–241. doi: 10.1093/infdis/143.2.238. [DOI] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Waddell A. Interferonlike factors from antigen- and mitogen-stimulated human leukocytes with antirickettsial and cytolytic actions on Rickettsia prowazekii. Infected human endothelial cells, fibroblasts, and macrophages. J Exp Med. 1983 Jun 1;157(6):1780–1793. doi: 10.1084/jem.157.6.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bel-Kahn J. M., Watanakunakorn C., Menefee M. G., Long H. D., Dicter R. Chlamydia trachomatis endocarditis. Am Heart J. 1978 May;95(5):627–636. doi: 10.1016/0002-8703(78)90305-8. [DOI] [PubMed] [Google Scholar]


