Abstract
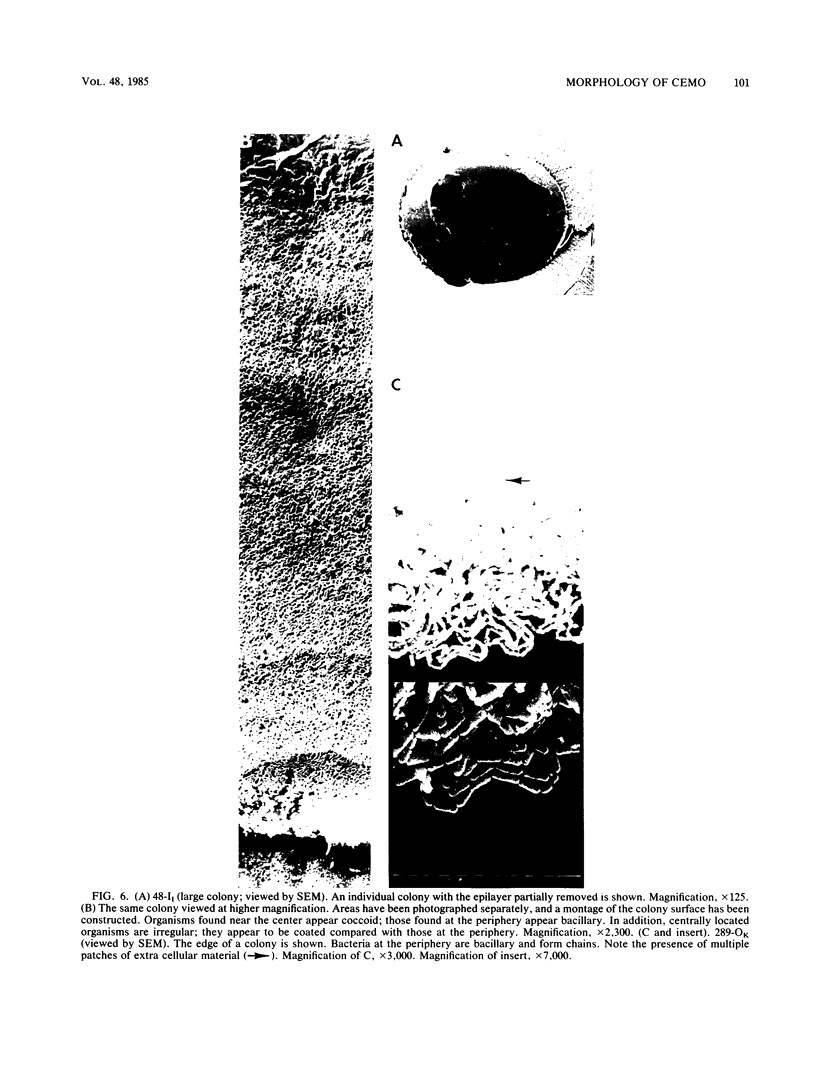
Examination of recently isolated cultures of three strains of Contagious Equine Metritis Organism grown on specially formulated, serum-free, clear typing medium revealed the presence of numerous colonial opacity variants. These colonies were prepared by a number of fixation and staining techniques and examined by scanning and transmission electron microscopy. Opaque and transparent phenotypes produced copious amounts of extracellular material compared with intermediate-opacity phenotypes which produced little or none. Also unique to intermediate colonies were numerous thin intercellular strands, which may represent pili or polymers of extracellular material. The presence of an unusual fibrillar layer (with similar electron density to the extracellular material) on the outer leaf of the outer membrane also was confirmed. A number of other ultrastructural features also were noted, including an epilayer, a thin nonmembranous layer which covered colonies and adjacent agar.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake M. S., Gotschlich E. C. Purification and partial characterization of the opacity-associated proteins of Neisseria gonorrhoeae. J Exp Med. 1984 Feb 1;159(2):452–462. doi: 10.1084/jem.159.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew K., Shaper J. H., Olsen K. W., Trayer I. P., Hill R. L. Cross-linking of the components of lactose synthetase with dimethylpimelimidate. J Biol Chem. 1975 Feb 25;250(4):1434–1444. [PubMed] [Google Scholar]
- Buchanan T. M. Antigenic heterogeneity of gonococcal pili. J Exp Med. 1975 Jun 1;141(6):1470–1475. doi: 10.1084/jem.141.6.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaglesome M. D., Garcia M. M. Contagious equine metritis: a review. Can Vet J. 1979 Aug;20(8):201–206. [PMC free article] [PubMed] [Google Scholar]
- Fernie D. S., Batty I., Walker P. D., Platt H., Mackintosh M. E., Simpson D. J. Observations on vaccine and post-infection immunity in contagious equine metritis. Res Vet Sci. 1980 May;28(3):362–367. [PubMed] [Google Scholar]
- Goldman R. C., White D., Orskov F., Orskov I., Rick P. D., Lewis M. S., Bhattacharjee A. K., Leive L. A surface polysaccharide of Escherichia coli O111 contains O-antigen and inhibits agglutination of cells by O-antiserum. J Bacteriol. 1982 Sep;151(3):1210–1221. doi: 10.1128/jb.151.3.1210-1221.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Ito S., Vinson J. W., McGuire T. J., Jr Murine typhus Rickettsiae in the Oriental rat flea. Ann N Y Acad Sci. 1975;266:35–60. doi: 10.1111/j.1749-6632.1975.tb35087.x. [DOI] [PubMed] [Google Scholar]
- James J. F., Swanson J. Studies on gonococcus infection. XIII. Occurrence of color/opacity colonial variants in clinical cultures. Infect Immun. 1978 Jan;19(1):332–340. doi: 10.1128/iai.19.1.332-340.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jephcott A. E., Reyn A., Birch-Andersen A. Neisseria gonorrhoeae 3. Demonstration of presumed appendages to cells from different colony types. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(3):437–439. doi: 10.1111/j.1699-0463.1971.tb00086.x. [DOI] [PubMed] [Google Scholar]
- Kellogg D. S., Jr, Cohen I. R., Norins L. C., Schroeter A. L., Reising G. Neisseria gonorrhoeae. II. Colonial variation and pathogenicity during 35 months in vitro. J Bacteriol. 1968 Sep;96(3):596–605. doi: 10.1128/jb.96.3.596-605.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAWN A. M. The use of potassium permanganate as an electron-dense stain for sections of tissue embedded in epoxy resin. J Biophys Biochem Cytol. 1960 Feb;7:197–198. doi: 10.1083/jcb.7.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambden P. R., Heckels J. E., James L. T., Watt P. J. Variations in surface protein composition associated with virulence properties in opacity types of Neisseria gonorrhoeae. J Gen Microbiol. 1979 Oct;114(2):305–312. doi: 10.1099/00221287-114-2-305. [DOI] [PubMed] [Google Scholar]
- Lambden P. R., Robertson J. N., Watt P. J. Biological properties of two distinct pilus types produced by isogenic variants of Neisseria gonorrhoeae P9. J Bacteriol. 1980 Jan;141(1):393–396. doi: 10.1128/jb.141.1.393-396.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- Malick L. E., Wilson R. B. Modified thiocarbohydrazide procedure for scanning electron microscopy: routine use for normal, pathological, or experimental tissues. Stain Technol. 1975 Jul;50(4):265–269. doi: 10.3109/10520297509117069. [DOI] [PubMed] [Google Scholar]
- Matsumoto A., Fujiwara E., Higashi N. Observations of the surface projections of infectious small cell of Chlamydia psittaci in thin sections. J Electron Microsc (Tokyo) 1976;25(3):169–170. [PubMed] [Google Scholar]
- Palumbo S. A., Johnson M. G., Rieck V. T., Witter L. D. Growth measurements on surface colonies of bacteria. J Gen Microbiol. 1971 May;66(2):137–143. doi: 10.1099/00221287-66-2-137. [DOI] [PubMed] [Google Scholar]
- Powell D. G. Contagious equine metritis. Adv Vet Sci Comp Med. 1981;25:161–184. [PubMed] [Google Scholar]
- Reyrolle J., Letellier F. Autoradiographic study of the localization and evolution of growth zones in bacterial colonies. J Gen Microbiol. 1979 Apr;111(2):399–406. doi: 10.1099/00221287-111-2-399. [DOI] [PubMed] [Google Scholar]
- Sahu S. P. Contagious equine metritis: effect of vaccination on control of the disease. Am J Vet Res. 1981 Jan;42(1):45–48. [PubMed] [Google Scholar]
- Sahu S. P., Dardiri A. H. Contagious equine metritis: isolation and characterization of the etiologic agent. Am J Vet Res. 1980 Sep;41(9):1379–1382. [PubMed] [Google Scholar]
- Sahu S. P., Weber S. Contagious equine metritis: effect of intrauterine inoculation of tiny colony forms in pony mares. Vet Rec. 1982 Mar 13;110(11):250–251. doi: 10.1136/vr.110.11.250. [DOI] [PubMed] [Google Scholar]
- Sahu S. P., Wool S., Breese S. S., Jr Observation on the morphology of contagious equine metritis bacterial colonies isolated from infected pony mares. Am J Vet Res. 1982 May;43(5):796–800. [PubMed] [Google Scholar]
- Salit I. E., Blake M., Gotschlich E. C. Intra-strain heterogeneity of gonococcal pili is related to opacity colony variance. J Exp Med. 1980 Mar 1;151(3):716–725. doi: 10.1084/jem.151.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood J. E., Vasse J. M., Dazzo F. B., Truchet G. L. Development and trifoliin A-binding ability of the capsule of Rhizobium trifolii. J Bacteriol. 1984 Jul;159(1):145–152. doi: 10.1128/jb.159.1.145-152.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M. T., Sousa J. C. Ultrastructure of the cell wall and cytoplasmic membrane of gram-negative bacteria with different fixation techniques. J Bacteriol. 1973 Feb;113(2):953–962. doi: 10.1128/jb.113.2.953-962.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Sutton J. S. Potassium permanganate staining of ultrathin sections for electron microscopy. J Ultrastruct Res. 1967 Dec;21(5):424–429. doi: 10.1016/s0022-5320(67)80150-3. [DOI] [PubMed] [Google Scholar]
- Swaney L. M., Breese S. S., Jr Ultrastructure of Haemophilus equigenitalis, causative agent of contagious equine metritis. Am J Vet Res. 1980 Jan;41(1):127–132. [PubMed] [Google Scholar]
- Swanson J., Barrera O. Gonococcal pilus subunit size heterogeneity correlates with transitions in colony piliation phenotype, not with changes in colony opacity. J Exp Med. 1983 Nov 1;158(5):1459–1472. doi: 10.1084/jem.158.5.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Colony opacity and protein II compositions of gonococci. Infect Immun. 1982 Jul;37(1):359–368. doi: 10.1128/iai.37.1.359-368.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Kraus S. J., Gotschlich E. C. Studies on gonococcus infection. I. Pili and zones of adhesion: their relation to gonococcal growth patterns. J Exp Med. 1971 Oct 1;134(4):886–906. doi: 10.1084/jem.134.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J Exp Med. 1973 Mar 1;137(3):571–589. doi: 10.1084/jem.137.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XIV. Cell wall protein differences among color/opacity colony variants of Neisseria gonorrhoeae. Infect Immun. 1978 Jul;21(1):292–302. doi: 10.1128/iai.21.1.292-302.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. E., Rosenthal R. O., Brown D. F., Lapage S. P., Hill L. R., Legros R. M. The causative organism of contagious equine metritis 1977: proposal for a new species to be known as Haemophilus equigenitalis. Equine Vet J. 1978 Jul;10(3):136–144. doi: 10.1111/j.2042-3306.1978.tb02242.x. [DOI] [PubMed] [Google Scholar]
- Timoney P. J., O'Reilly P. J., McArdle J. F., Ward J., Harrington A. M., McCormack R. Responses of pony mares to the agent of contagious equine metritis 1977. J Reprod Fertil Suppl. 1979;(27):367–375. [PubMed] [Google Scholar]
- Timoney P. J., O'Reilly P. J., McArdle J. F., Ward J., Harrington A. M. Responses of mares to rechallenge with the organism of contagious equine metritis 1977. Vet Rec. 1979 Mar 24;104(12):264–264. doi: 10.1136/vr.104.12.264. [DOI] [PubMed] [Google Scholar]
- Timoney P. J., Shin S. J., Jacobson R. H. Improved selective medium for isolation of the contagious equine metritis organism. Vet Rec. 1982 Jul 31;111(5):107–108. doi: 10.1136/vr.111.5.107. [DOI] [PubMed] [Google Scholar]
- Todd W. J., Wray G. P., Hitchcock P. J. Arrangement of pili in colonies of Neisseria gonorrhoeae. J Bacteriol. 1984 Jul;159(1):312–320. doi: 10.1128/jb.159.1.312-320.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walstad D. L., Guymon L. F., Sparling P. F. Altered outer membrane protein in different colonial types of Neisseria gonorrhoeae. J Bacteriol. 1977 Mar;129(3):1623–1627. doi: 10.1128/jb.129.3.1623-1627.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]