Abstract
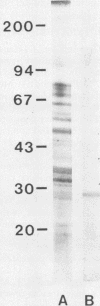
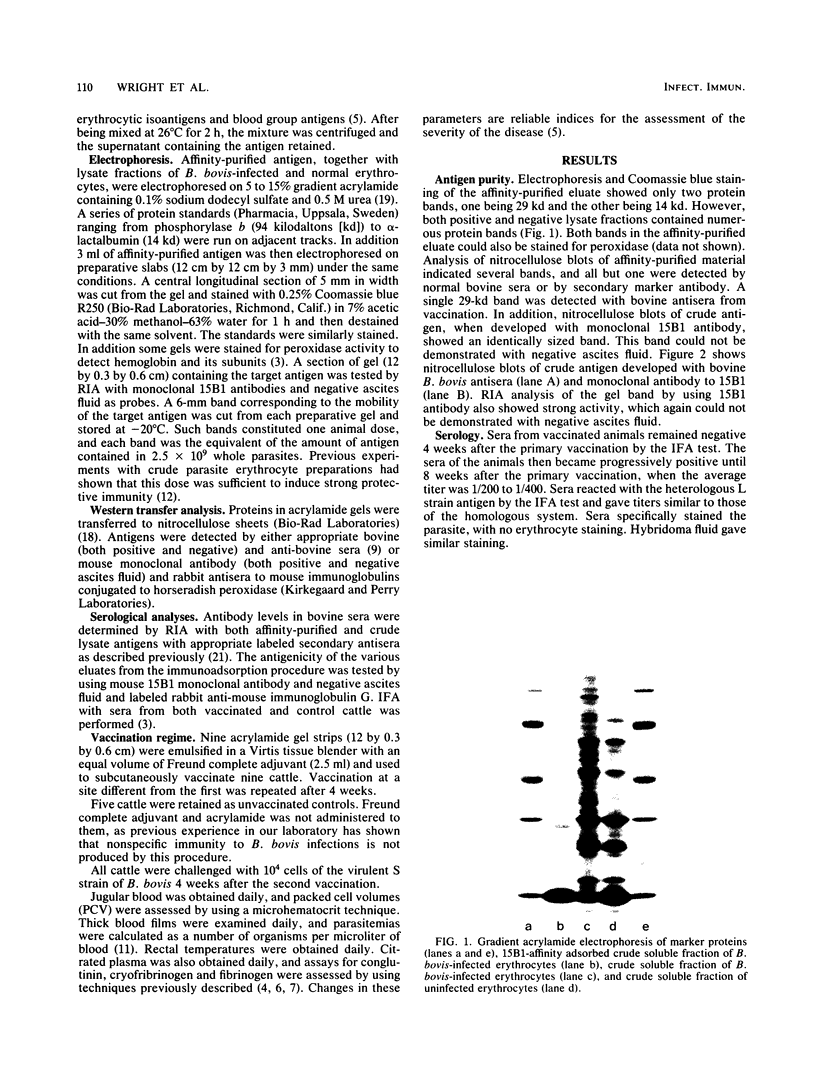
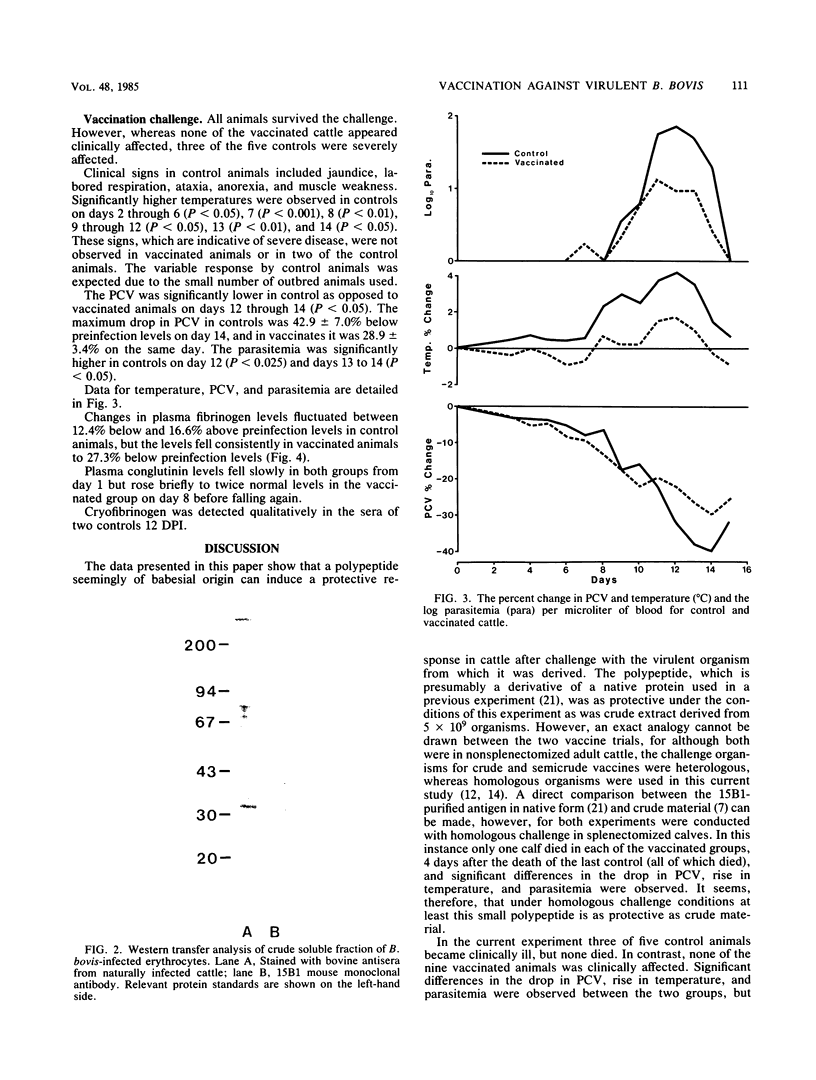
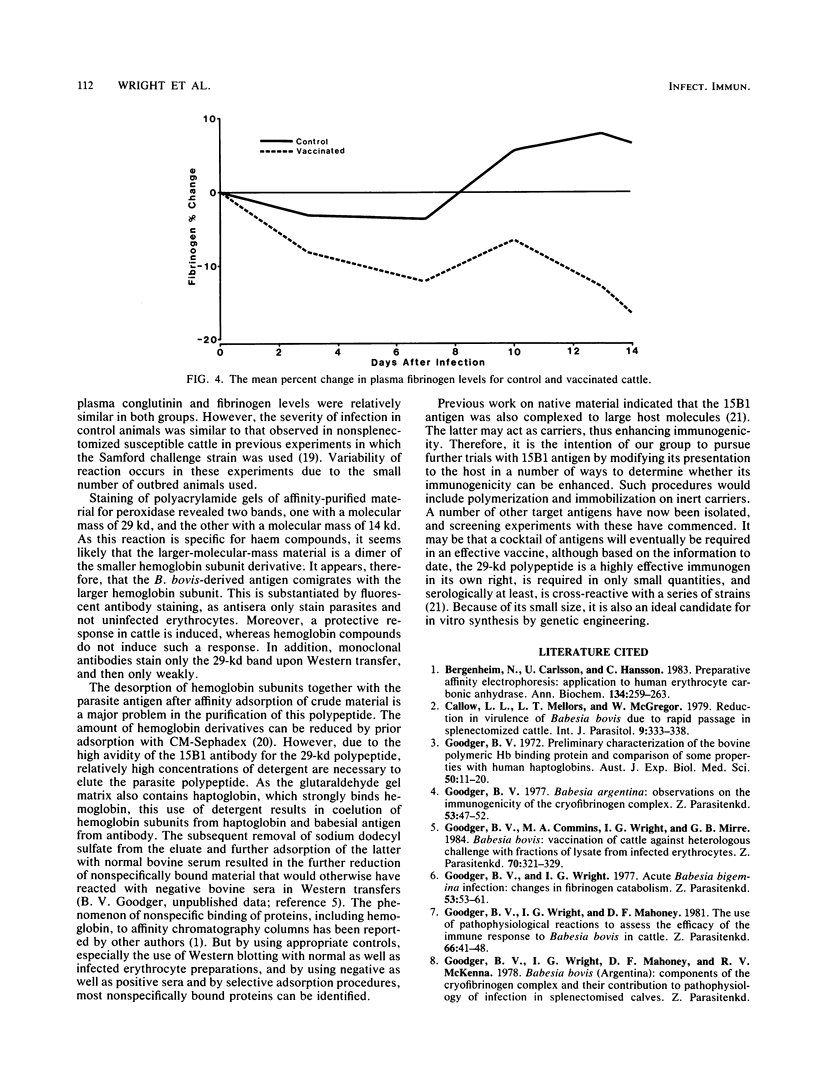
A Babesia bovis low-molecular-weight antigen was purified from crude material by using affinity adsorption techniques first with a mouse monoclonal antibody and then by adsorption with normal bovine sera. The antigen was then further purified by gradient gel electrophoresis. Analysis by Western transfer revealed only one antigen band, with an apparent molecular mass of 29 kilodaltons. A band (12 by 0.3 by 0.6 cm) corresponding to this antigen was excised from the acrylamide gel and injected twice 4 weeks apart, together with 2.5 ml of Freund complete adjuvant, into nine nonsplenectomized adult cattle. The vaccinated cattle and five susceptible control animals were challenged with a virulent homologous strain 4 weeks after the second vaccination. None of the vaccinated animals was clinically affected, whereas three of five controls were severely affected. Control animals had significantly greater declines in packed cell volume and greater rises in temperature and parasitaemias than vaccinated animals.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergenhem N., Carlsson U., Hansson C. Preparative affinity electrophoresis: application to human erythrocyte carbonic anhydrase. Anal Biochem. 1983 Oct 1;134(1):259–263. doi: 10.1016/0003-2697(83)90294-4. [DOI] [PubMed] [Google Scholar]
- Callow L. L., Mellors L. T., McGregor W. Reduction in virulence of Babesia bovis due to rapid passage in splenectomized cattle. Int J Parasitol. 1979 Aug;9(4):333–338. doi: 10.1016/0020-7519(79)90083-3. [DOI] [PubMed] [Google Scholar]
- Goodger B. V. Babesia argentina: observations on the immunogenicity of the cryofibrinogen complex. Z Parasitenkd. 1977 Aug 25;53(1):47–52. doi: 10.1007/BF00383114. [DOI] [PubMed] [Google Scholar]
- Goodger B. V., Commins M. A., Wright I. G., Mirre G. B. Babesia bovis: vaccination of cattle against heterologous challenge with fractions of lysate from infected erythrocytes. Z Parasitenkd. 1984;70(3):321–329. doi: 10.1007/BF00927818. [DOI] [PubMed] [Google Scholar]
- Goodger B. V. Preliminary characterization of the bovine polymeric Hb binding protein and comparison of some properties with human haptoglobins. Aust J Exp Biol Med Sci. 1972 Feb;50(1):11–20. doi: 10.1038/icb.1972.2. [DOI] [PubMed] [Google Scholar]
- Goodger B. V., Wright I. G. Acute Babesia bigemina infection: changes in fibrinogen catabolism. Z Parasitenkd. 1977 Aug 25;53(1):53–61. doi: 10.1007/BF00383115. [DOI] [PubMed] [Google Scholar]
- Goodger B. V., Wright I. G., Mahoney D. F. The use of pathophysiological reactions to assess the efficacy of the immune response to Babesia bovis in cattle. Z Parasitenkd. 1981;66(1):41–48. doi: 10.1007/BF00941944. [DOI] [PubMed] [Google Scholar]
- Goodger B. V., Wright I. G., Waltisbuhl D. J. The lysate from bovine erythrocytes infected with Babesia bovis. Analysis of antigens and a report on their immunogenicity when polymerized with glutaraldehyde. Z Parasitenkd. 1983;69(4):473–482. doi: 10.1007/BF00927703. [DOI] [PubMed] [Google Scholar]
- Kuttler K. L., Levy M. G., James M. A., Ristic M. Efficacy of a nonviable culture-derived Babesia bovis vaccine. Am J Vet Res. 1982 Feb;43(2):281–284. [PubMed] [Google Scholar]
- Mahoney D. F., Wright I. G., Goodger B. V. Bovine babesiosis: the immunization of cattle with fractions of erythrocytes infected with Babesia bovis (syn B. argentina). Vet Immunol Immunopathol. 1981 Apr;2(2):145–156. doi: 10.1016/0165-2427(81)90046-5. [DOI] [PubMed] [Google Scholar]
- Mahoney D. F., Wright I. G., Goodger B. V. Immunity in cattle to Babesia bovis after single infections with parasites of various origin. Aust Vet J. 1979 Jan;55(1):10–12. doi: 10.1111/j.1751-0813.1979.tb09535.x. [DOI] [PubMed] [Google Scholar]
- Mahoney D. F., Wright I. G., Ketterer P. J. Babesia argentina: the infectivity and immunogenicity of irradiated blood parasites for splenectomized calves. Int J Parasitol. 1973 Mar;3(2):209–217. doi: 10.1016/0020-7519(73)90026-x. [DOI] [PubMed] [Google Scholar]
- Timms P., Dalgliesh R. J., Barry D. N., Dimmock C. K., Rodwell B. J. Babesia bovis: comparison of culture-derived parasites, non-living antigen and conventional vaccine in the protection of cattle against heterologous challenge. Aust Vet J. 1983 Mar;60(3):75–77. doi: 10.1111/j.1751-0813.1983.tb05874.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright I. G., Goodger B. V., Rode-Bramanis K., Mattick J. S., Mahoney D. F., Waltisbuhl D. J. The characterisation of an esterase derived from Babesia bovis and its use as a vaccine. Z Parasitenkd. 1983;69(6):703–714. doi: 10.1007/BF00927420. [DOI] [PubMed] [Google Scholar]
- Wright I. G., Mahoney D. F., Mirre G. B., Goodger B. V., Kerr J. D. The irradiation of babesia bovis. II. The immunogenicity of irradiated blood parasites for intact cattle and splenectomised calves. Vet Immunol Immunopathol. 1982 Nov;3(6):591–601. doi: 10.1016/0165-2427(82)90042-3. [DOI] [PubMed] [Google Scholar]
- Wright I. G., White M., Tracey-Patte P. D., Donaldson R. A., Goodger B. V., Waltisbuhl D. J., Mahoney D. F. Babesia bovis: isolation of a protective antigen by using monoclonal antibodies. Infect Immun. 1983 Jul;41(1):244–250. doi: 10.1128/iai.41.1.244-250.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]