Abstract
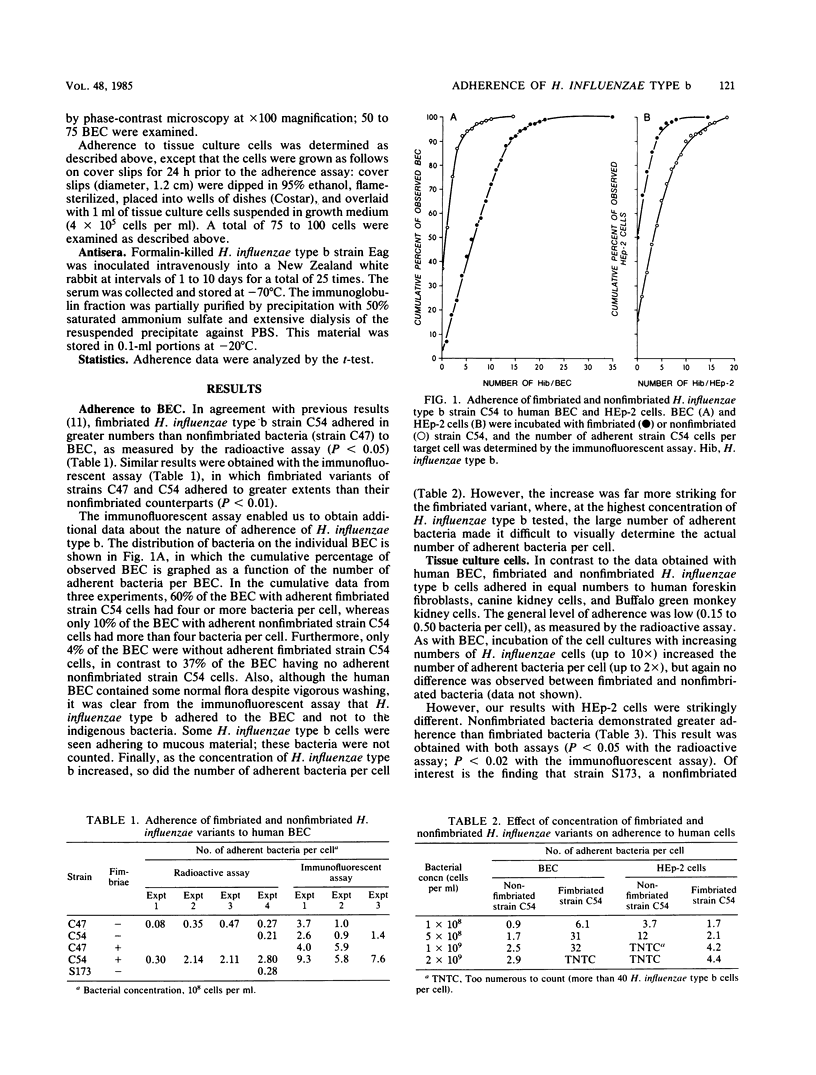
The attachment of isogenic fimbriated and nonfimbriated Haemophilus influenzae type b variants to human cells was studied by using a radioactive assay and an indirect immunofluorescent assay. As described previously, fimbriated H. influenzae variants adhered to a greater extent than nonfimbriated variants to human buccal epithelial cells (2.1 and 0.29 bacteria per cell, respectively, as determined by the radioactive assay [P less than 0.05]; 7.6 and 1.6 bacteria per cell, respectively, as determined by the immunofluorescent assay [P less than 0.01]). As the concentration of fimbriated bacteria was increased, so were the numbers of adherent bacteria; in contrast, increasing the bacterial concentration had a much smaller effect on adherence of nonfimbriated H. influenzae type b. The distribution of bacteria on the buccal cells also differed. Whereas 37% of the buccal cells failed to bind nonfimbriated H. influenzae type b, failure to bind was observed for only 4% of the buccal cells exposed to fimbriated H. influenzae. In contrast, adherence to human foreskin fibroblasts was low regardless of the presence of fimbriae. On the other hand, fimbriated H. influenzae type b adhered less well than nonfimbriated variants to HEp-2 cells (1.6 and 3.8 bacteria per cell, respectively, as determined by the radioactive assay [P less than 0.05]; 1.3 and 4.8 bacteria per cell, respectively, as determined by the immunofluorescent assay [P less than 0.02]). Whereas adherence to HEp-2 cells increased considerably as the concentration of nonfimbriated bacteria was increased, there was only a small enhancement of adherence with an increase in the concentration of fimbriated H. influenzae type b. Furthermore, only 16% of the HEp-2 cells failed to bind nonfimbriated H. influenzae type b, whereas 50% failed to bind fimbriated H. influenzae type b. These data indicate that H. influenzae type b may contain two adhesins. One is associated with fimbriae and enables adherence to buccal cells, whereas the other is nonfimbrial and is associated with adherence to HEp-2 cells. It is not known whether either of these adhesins plays a role in pathogenesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barenkamp S. J., Granoff D. M., Munson R. S., Jr Outer-membrane protein subtypes of Haemophilus influenzae type b and spread of disease in day-care centers. J Infect Dis. 1981 Sep;144(3):210–217. doi: 10.1093/infdis/144.3.210. [DOI] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Connor E. M., Loeb M. R. A hemadsorption method for detection of colonies of Haemophilus influenzae type b expressing fimbriae. J Infect Dis. 1983 Nov;148(5):855–860. doi: 10.1093/infdis/148.5.855. [DOI] [PubMed] [Google Scholar]
- Guerina N. G., Langermann S., Clegg H. W., Kessler T. W., Goldman D. A., Gilsdorf J. R. Adherence of piliated Haemophilus influenzae type b to human oropharyngeal cells. J Infect Dis. 1982 Oct;146(4):564–564. doi: 10.1093/infdis/146.4.564. [DOI] [PubMed] [Google Scholar]
- Kaplan S. L., Mason E. O., Jr, Wiedermann B. L. Role of adherence in the pathogenesis of Haemophilus influenzae type b infection in infant rats. Infect Immun. 1983 Nov;42(2):612–617. doi: 10.1128/iai.42.2.612-617.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Outer membrane protein composition in disease isolates of Haemophilus influenzae: pathogenic and epidemiological implications. Infect Immun. 1980 Dec;30(3):709–717. doi: 10.1128/iai.30.3.709-717.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M. R., Zachary A. L., Smith D. H. Isolation and partial characterization of outer and inner membranes from encapsulated Haemophilus influenzae type b. J Bacteriol. 1981 Jan;145(1):596–604. doi: 10.1128/jb.145.1.596-604.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old D. C., Robertson J. Adherence of fimbriate and non-fimbriate strains of Yersinia enterocolitica to human epithelial cells. Microbiol Immunol. 1981;25(10):993–998. doi: 10.1111/j.1348-0421.1981.tb00105.x. [DOI] [PubMed] [Google Scholar]
- Pichichero M. E., Loeb M., Anderson, Smith D. H. Do pili play a role in pathogenicity of Haemophilus influenzae type B? Lancet. 1982 Oct 30;2(8305):960–962. doi: 10.1016/s0140-6736(82)90161-1. [DOI] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Type I Escherichia coli pili: characterization of binding to monkey kidney cells. J Exp Med. 1977 Nov 1;146(5):1182–1194. doi: 10.1084/jem.146.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavendale A., Jardine C. K., Old D. C., Duguid J. P. Haemagglutinins and adhesion of Salmonella typhimurium to HEp2 and HeLa cells. J Med Microbiol. 1983 Aug;16(3):371–380. doi: 10.1099/00222615-16-3-371. [DOI] [PubMed] [Google Scholar]


