Abstract
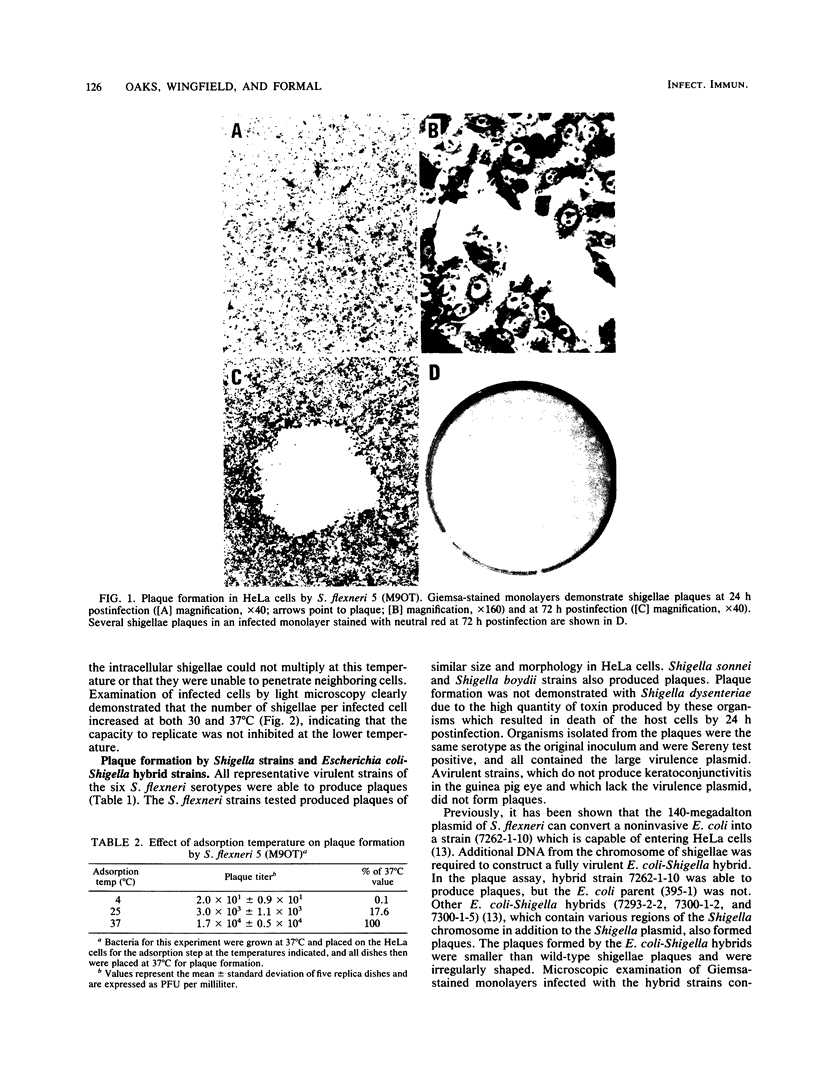
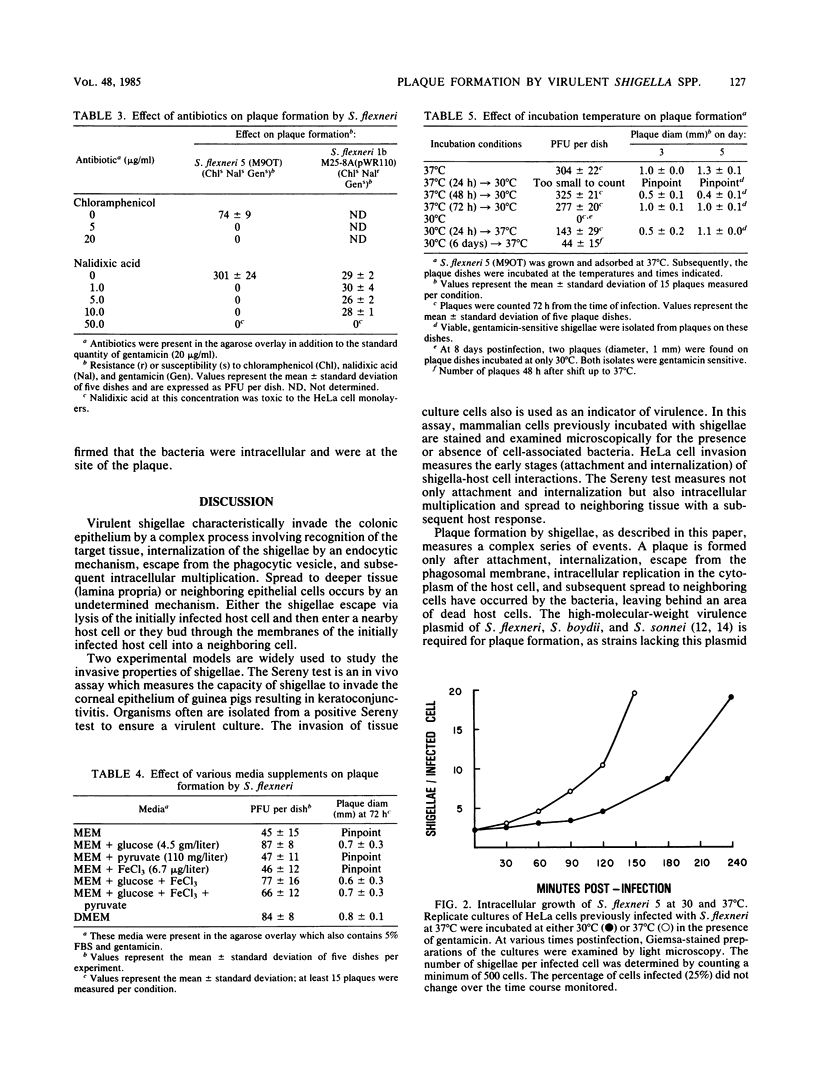
An in vitro tissue culture plaque assay was developed to investigate the intracellular replication and intercellular spread of virulent shigellae. Shigella plaques were formed in HeLa cell monolayers in the presence of an agarose overlay containing tissue culture medium and gentamicin, which eliminated extracellular bacterial growth. Microscopically, the plaques were characterized by a central area of dead host cells surrounded by cells infected with shigellae. Cells further away from the plaque center were uninfected. Inclusion of chloramphenicol or nalidixic acid in the overlay completely abolished plaque formation. Plaque formation was completely inhibited when infected monolayers were shifted from 37 to 30 degrees C. Shifting infected monolayers from 30 degrees C, where plaques do not form, to 37 degrees C resulted in the formation of plaques. Cultures of Shigella boydii, Shigella sonnei (form I), and all six serotypes of Shigella flexneri produced plaques. Shigellae isolated from plaques were Sereny test positive, contained a 140-megadalton plasmid, and were gentamicin sensitive. Noninvasive shigellae did not form plaques.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FORMAL S. B., LABREC E. H., KENT T. H., FALKOW S. ABORTIVE INTESTINAL INFECTION WITH AN ESCHERICHIA COLI-SHIGELLA FLEXNERI HYBRID STRAIN. J Bacteriol. 1965 May;89:1374–1382. doi: 10.1128/jb.89.5.1374-1382.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Bonventre P. F. Shigella infection of Henle intestinal epithelial cells: role of the bacterium. Infect Immun. 1979 Jun;24(3):879–886. doi: 10.1128/iai.24.3.879-886.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Formal S. B. Cytotoxicity of Shigella dysenteriae 1 for cultured mammalian cells. Am J Clin Nutr. 1980 Nov;33(11 Suppl):2485–2490. doi: 10.1093/ajcn/33.11.2485. [DOI] [PubMed] [Google Scholar]
- Hale T. L., Formal S. B. Protein synthesis in HeLa or Henle 407 cells infected with Shigella dysenteriae 1, Shigella flexneri 2a, or Salmonella typhimurium W118. Infect Immun. 1981 Apr;32(1):137–144. doi: 10.1128/iai.32.1.137-144.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Morris R. E., Bonventre P. F. Shigella infection of henle intestinal epithelial cells: role of the host cell. Infect Immun. 1979 Jun;24(3):887–894. doi: 10.1128/iai.24.3.887-894.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihlström E. Infection of HeLa cells with Salmonella typhimurium 395 MS and MR10 bacteria. Infect Immun. 1977 Aug;17(2):290–295. doi: 10.1128/iai.17.2.290-295.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecko D. J., Washington O., Formal S. B. Genetic and physical evidence for plasmid control of Shigella sonnei form I cell surface antigen. Infect Immun. 1980 Jul;29(1):207–214. doi: 10.1128/iai.29.1.207-214.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrec E. H., Schneider H., Magnani T. J., Formal S. B. EPITHELIAL CELL PENETRATION AS AN ESSENTIAL STEP IN THE PATHOGENESIS OF BACILLARY DYSENTERY. J Bacteriol. 1964 Nov;88(5):1503–1518. doi: 10.1128/jb.88.5.1503-1518.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo M. C., Mandell G. L. The effect of antibiotics on Escherichia coli ingested by macrophages. Proc Soc Exp Biol Med. 1973 Mar;142(3):1048–1050. doi: 10.3181/00379727-142-37173. [DOI] [PubMed] [Google Scholar]
- Maurelli A. T., Blackmon B., Curtiss R., 3rd Temperature-dependent expression of virulence genes in Shigella species. Infect Immun. 1984 Jan;43(1):195–201. doi: 10.1128/iai.43.1.195-201.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SERENY B. Experimental shigella keratoconjunctivitis; a preliminary report. Acta Microbiol Acad Sci Hung. 1955;2(3):293–296. [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982 Mar;35(3):852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., d'Hauteville H., Ecobichon C., Pourcel C. Molecular comparison of virulence plasmids in Shigella and enteroinvasive Escherichia coli. Ann Microbiol (Paris) 1983 May-Jun;134A(3):295–318. [PubMed] [Google Scholar]
- Takeuchi A., Formal S. B., Sprinz H. Exerimental acute colitis in the Rhesus monkey following peroral infection with Shigella flexneri. An electron microscope study. Am J Pathol. 1968 Mar;52(3):503–529. [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Sprinz H., LaBrec E. H., Formal S. B. Experimental bacillary dysentery. An electron microscopic study of the response of the intestinal mucosa to bacterial invasion. Am J Pathol. 1965 Dec;47(6):1011–1044. [PMC free article] [PubMed] [Google Scholar]



