Abstract
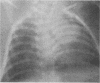
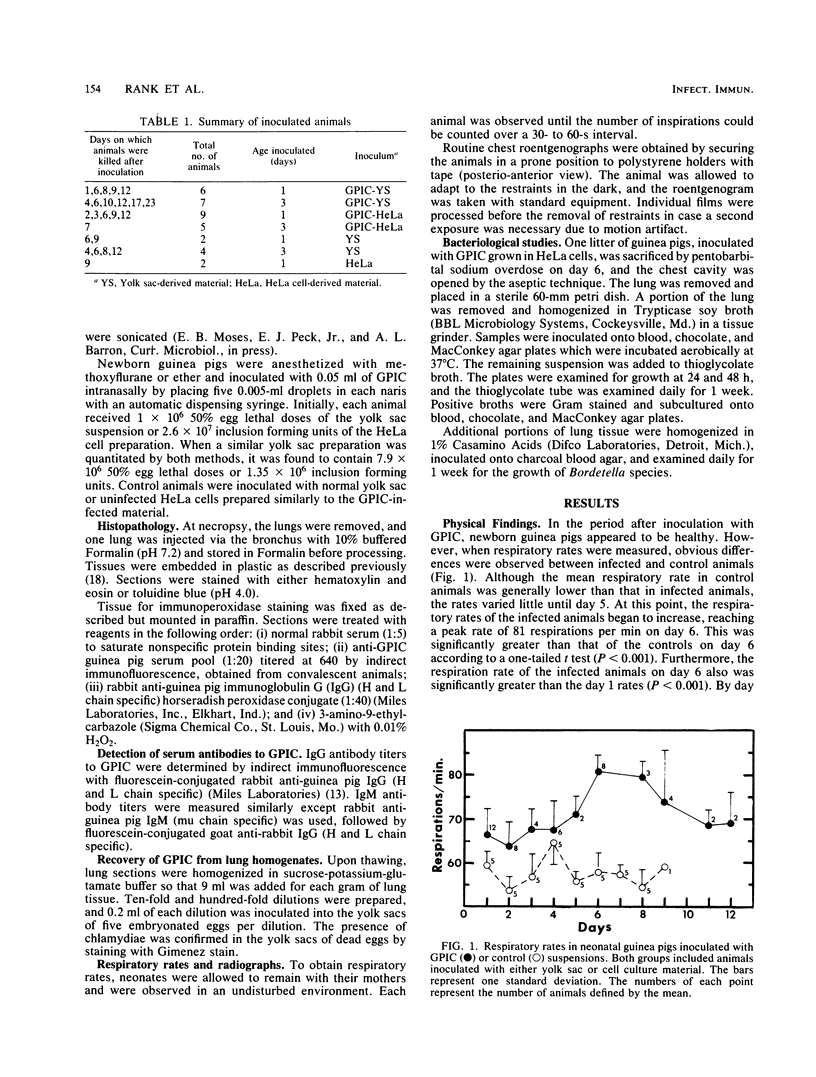
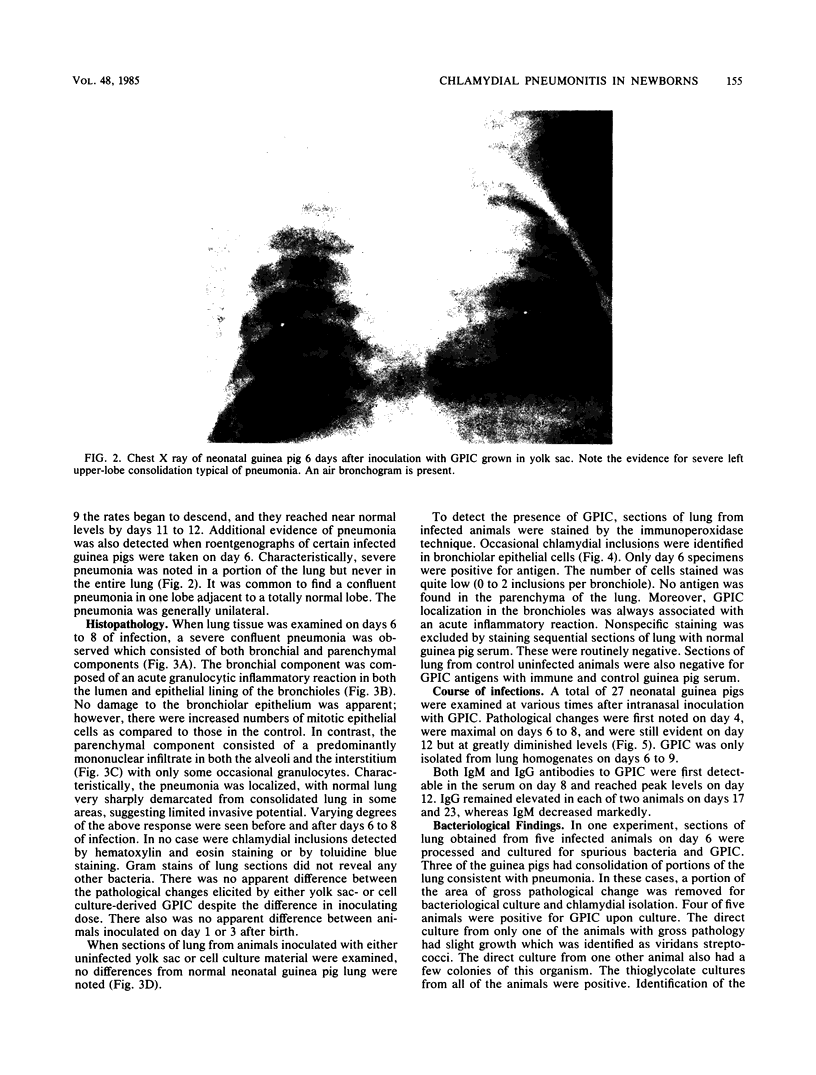
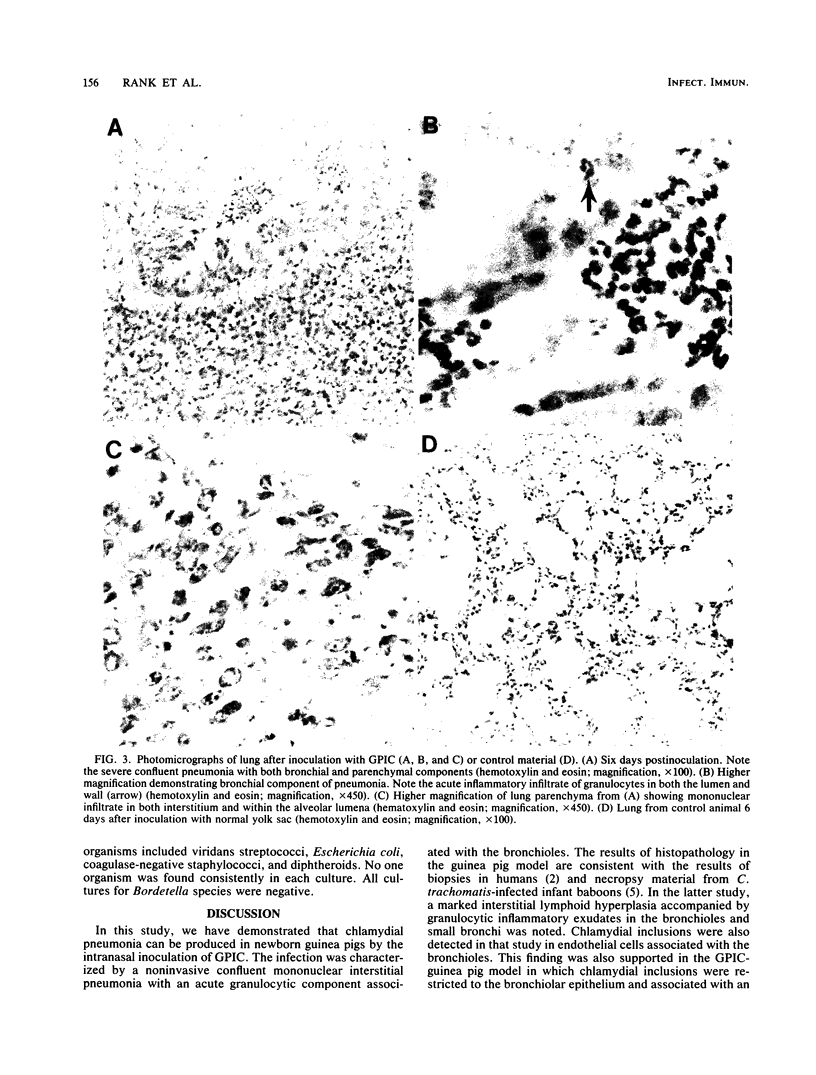
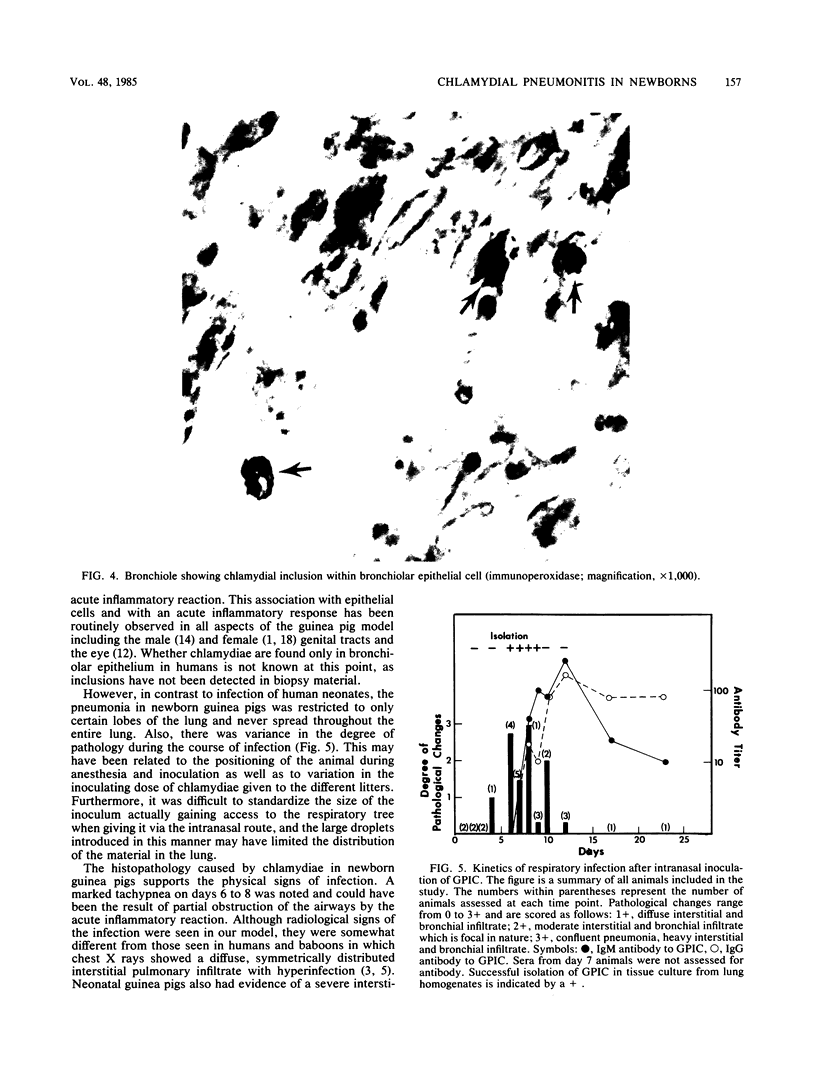
One- to three-day-old guinea pigs were inoculated intranasally with the chlamydial agent of guinea pig inclusion conjunctivitis. Physical signs of infection included a marked increase in respiration rate on days 5 to 10 of infection and radiographic evidence of pneumonia on day 6. When animals were killed at various times after infection and lung tissue was examined by histopathology, evidence of pneumonia was found beginning on day 4 and lasting as long as day 12, with maximal pathological changes on days 6 to 8. The pneumonia was generally unilateral and consisted of an acute inflammatory component in the bronchioles with granulocytes in both the lumen and the wall of the bronchioles and an interstitial and intra-alveolar mononuclear infiltrate in the parenchyma of the lung. Chlamydial antigen was detected in the bronchial epithelial cells by immunoperoxidase staining, and the guinea pig inclusion conjunctivitis organism was isolated from lung tissue on days 6 to 9. No other significant bacteria were isolated from lung tissue or seen on gram stains of lung sections. Both immunoglobulin M and immunoglobulin G serum antibodies to the guinea pig inclusion conjunctivitis agent were detected as early as day 8 and reached peak levels on day 12. The infection was apparently self-limiting. This model presents the opportunity to investigate pathophysiological and immunological aspects of chlamydial respiratory infections in a neonatal animal.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barron A. L., White H. J., Rank R. G., Soloff B. L. Target tissues associated with genital infection of female guinea pigs by the chlamydial agent of guinea pig inclusion conjunctivitis. J Infect Dis. 1979 Jan;139(1):60–68. doi: 10.1093/infdis/139.1.60. [DOI] [PubMed] [Google Scholar]
- Beem M. O., Saxon E. M. Respiratory-tract colonization and a distinctive pneumonia syndrome in infants infected with Chlamydia trachomatis. N Engl J Med. 1977 Feb 10;296(6):306–310. doi: 10.1056/NEJM197702102960604. [DOI] [PubMed] [Google Scholar]
- Harrison H. R., Alexander E. R., Chiang W. T., Giddens W. E., Jr, Boyce J. T., Benjamin D., Gale J. L. Experimental nasopharyngitis and pneumonia caused by Chlamydia trachomatis in infant baboons: histopathologic comparison with a case in a human infant. J Infect Dis. 1979 Feb;139(2):141–146. doi: 10.1093/infdis/139.2.141. [DOI] [PubMed] [Google Scholar]
- Harrison H. R., English M. G., Lee C. K., Alexander E. R. Chlamydia trachomatis infant pneumonitis: comparison with matched controls and other infant pneumonitis. N Engl J Med. 1978 Mar 30;298(13):702–708. doi: 10.1056/NEJM197803302981303. [DOI] [PubMed] [Google Scholar]
- Harrison H. R., Lee S. M., Lucas D. O. Chlamydia trachomatis pneumonitis in the C57BL/KsJ mouse: pathologic and immunologic features. J Lab Clin Med. 1982 Dec;100(6):953–962. [PubMed] [Google Scholar]
- Kuo C., Chen W. J. A mouse model of Chlamydia trachomatis pneumonitis. J Infect Dis. 1980 Feb;141(2):198–202. doi: 10.1093/infdis/141.2.198. [DOI] [PubMed] [Google Scholar]
- Lechner A. J., Banchero N. Advanced pulmonary development in newborn guinea pigs (Cavia porcellus). Am J Anat. 1982 Mar;163(3):235–246. doi: 10.1002/aja.1001630304. [DOI] [PubMed] [Google Scholar]
- MURRAY E. S. GUINEA PIG INCLUSION CONJUNCTIVITIS VIRUS. I. ISOLATION AND IDENTIFICATION AS A MEMBER OF THE PSITTACOSIS-LYMPHOGRANULOMA-TRACHOMA GROUP. J Infect Dis. 1964 Feb;114:1–12. doi: 10.1093/infdis/114.1.1. [DOI] [PubMed] [Google Scholar]
- Mount D. T., Bigazzi P. E., Barron A. L. Experimental genital infection of male guinea pigs with the agent of guinea pig inclusion conjunctivitis and transmission to females. Infect Immun. 1973 Dec;8(6):925–930. doi: 10.1128/iai.8.6.925-930.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount D. T., Bigazzi P. E., Barron A. L. Infection of genital tract and transmission of ocular infection to newborns by the agent of guinea pig inclusion conjunctivitis. Infect Immun. 1972 Jun;5(6):921–926. doi: 10.1128/iai.5.6.921-926.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank R. G., White H. J., Barron A. L. Humoral immunity in the resolution of genital infection in female guinea pigs infected with the agent of guinea pig inclusion conjunctivitis. Infect Immun. 1979 Nov;26(2):573–579. doi: 10.1128/iai.26.2.573-579.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank R. G., White H. J., Soloff B. L., Barron A. L. Cystitis associated with chlamydial infection of the genital tract in male guinea pigs. Sex Transm Dis. 1981 Jul-Sep;8(3):203–210. doi: 10.1097/00007435-198107000-00006. [DOI] [PubMed] [Google Scholar]
- Schachter J., Grossman M., Azimi P. H. Serology of Chlamydia trachomatis in infants. J Infect Dis. 1982 Oct;146(4):530–535. doi: 10.1093/infdis/146.4.530. [DOI] [PubMed] [Google Scholar]
- Tipple M. A., Beem M. O., Saxon E. M. Clinical characteristics of the afebrile pneumonia associated with Chlamydia trachomatis infection in infants less than 6 months of age. Pediatrics. 1979 Feb;63(2):192–197. [PubMed] [Google Scholar]
- White H. J., Rank R. G., Soloff B. L., Barron A. L. Experimental chlamydial salpingitis in immunosuppressed guinea pigs infected in the genital tract with the agent of guinea pig inclusion conjunctivitis. Infect Immun. 1979 Nov;26(2):728–735. doi: 10.1128/iai.26.2.728-735.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. M., Schachter J., Drutz D. J., Sumaya C. V. Pneumonia due to Chlamydia trachomatis in the immunocompromised (nude) mouse. J Infect Dis. 1981 Feb;143(2):238–241. doi: 10.1093/infdis/143.2.238. [DOI] [PubMed] [Google Scholar]