Abstract
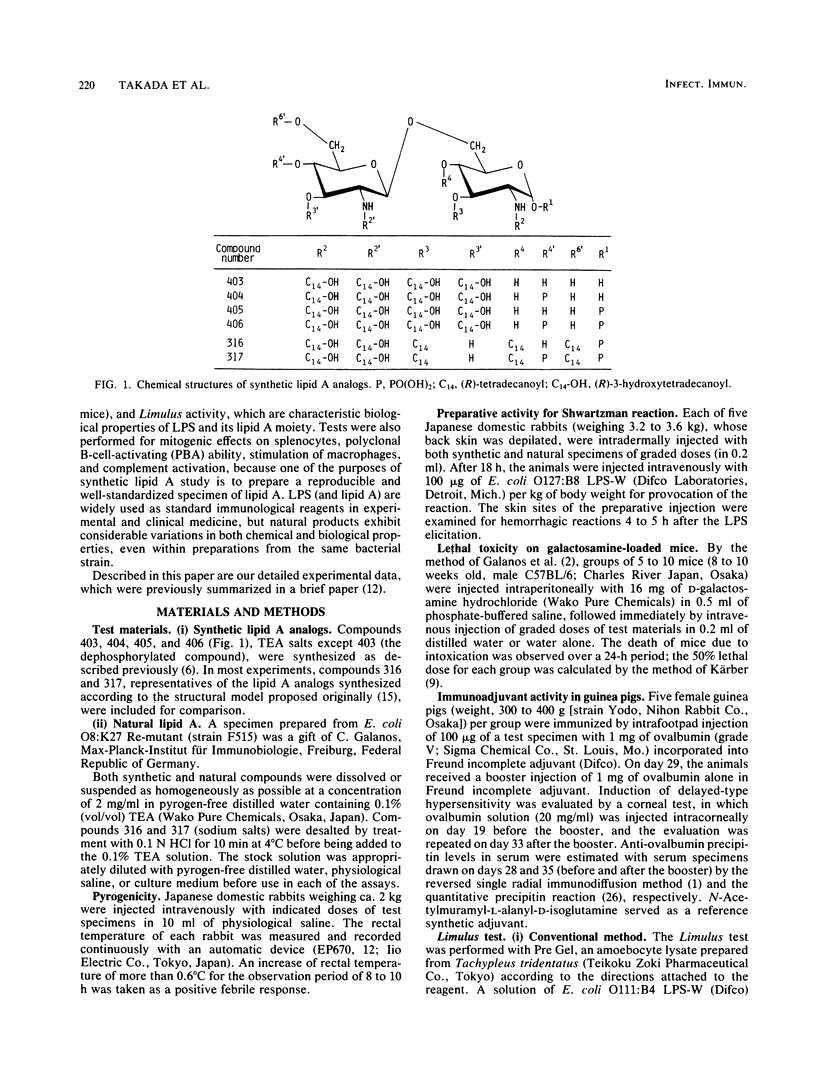
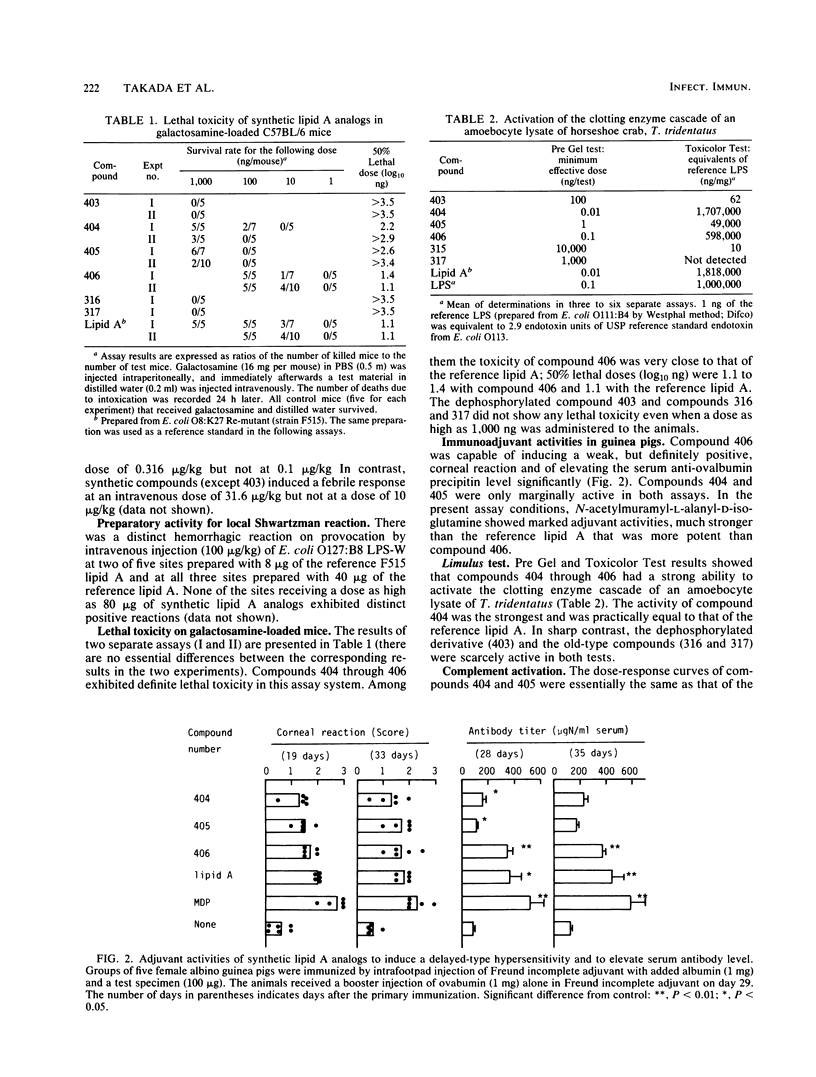
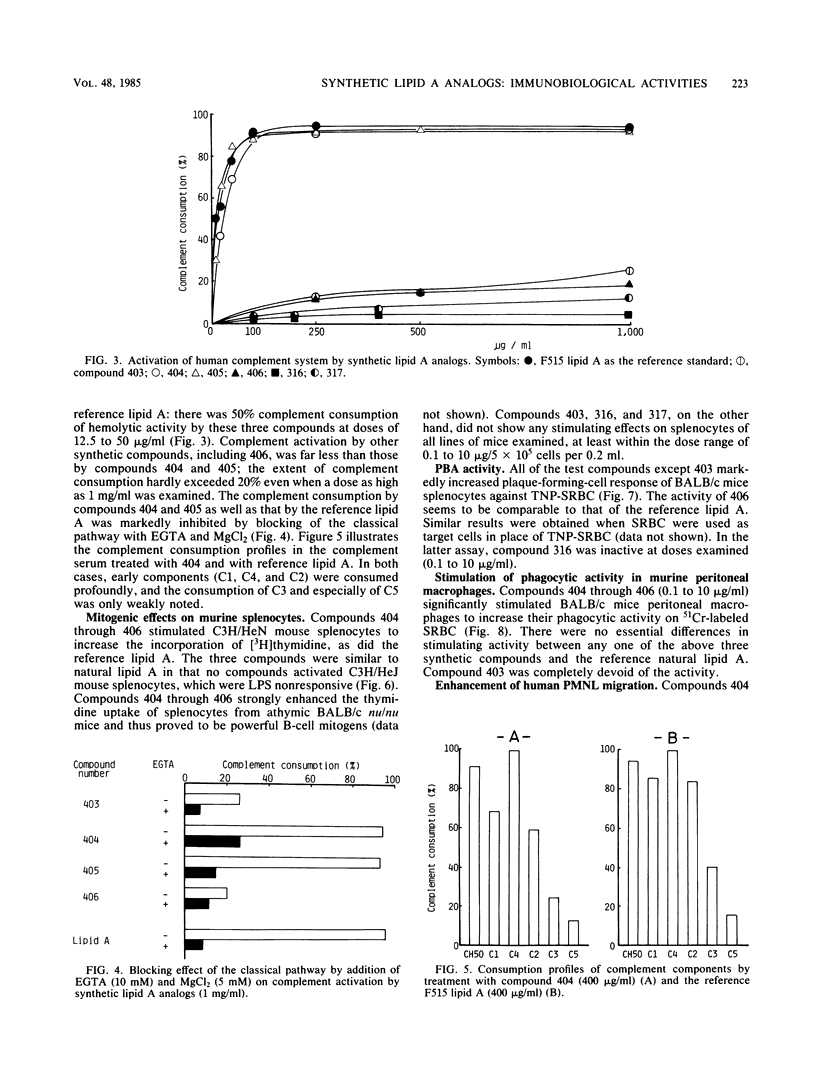
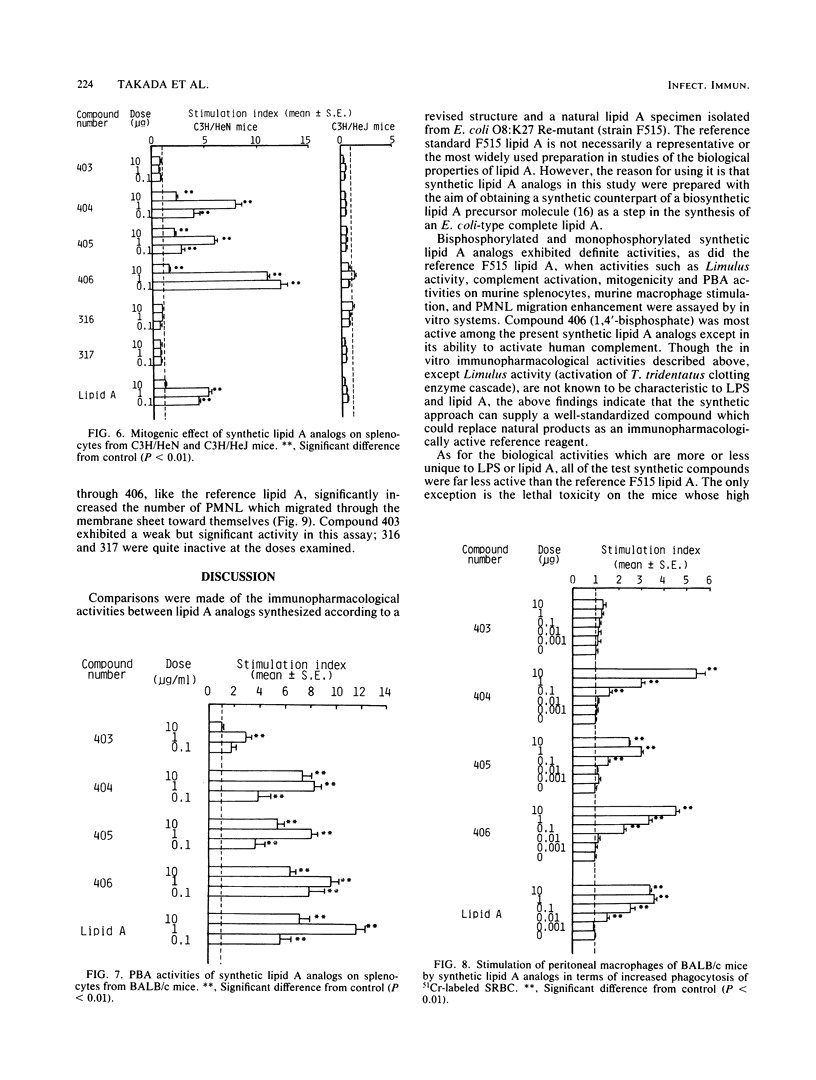
Synthetic lipid A analogs (compounds 404 through 406) were examined for their immunopharmacological activities. These compounds had two amide-bound and two ester-bound (R)-3-hydroxytetradecanoyl groups at the C-2 and C-2' and the C-3 and C-3' positions, respectively, of beta (1-3)glucosamine disaccharide. In all of the in vitro assays, these synthetic compounds exhibited high activities comparable to those of a reference lipid A prepared from Escherichia coli O8:K27 Re-mutant strain F515. The compounds activated the clotting enzyme cascade of the horseshoe crab, activated the human complement via the classical pathway, caused polyclonal B-cell activation, stimulated the phagocytosis of sheep erythrocytes by murine peritoneal macrophages, and enhanced the migration of human polymorphonuclear leukocytes. They also increased the thymidine uptake of splenocytes of BALB/c nu/nu and C3H/HeN mice but not those of C3H/HeJ (a nonresponder to lipopolysaccharide). A dephosphorylated derivative, compound 403, was barely active in all of the above assays except for the enhancement of polymorphonuclear leukocyte migration. However, compounds 404 through 406 were feeble in pyrogenicity and could not prepare the local Shwartzman reaction, although they were very lethal to galactosamine-loaded mice. Therefore, synthetic lipid A analogs described here were fully immunopharmacologically active in in vitro assays, but all of them were far less active than natural E. coli F515 lipid A regarding the biological activities characteristic of endotoxic lipopolysaccharides and lipid A's. The high lethal toxicity of compound 406 (1,4'-bisphosphate) to the galactosamine-loaded mice may not reflect its real toxicity to normal mice. In all activities examined, compound 406 was quite comparable to a biosynthetic lipid A precursor, a natural counterpart of compound 406. The immunopharmacological activities of these newly synthesized lipid A analogs, especially compound 406, were much stronger than those of compounds that had been synthesized earlier by using the originally proposed model of the lipid A structure. The findings described in this report justify the acylation pattern of a disaccharide backbone of lipid A, revised on the basis of recent analytical studies. The low in vivo endotoxic activities of the present lipid A analogs are most probably due to the fact that the kinds of acyl groups were different from those of the complete lipid A from E. coli, although there were no differences in the acylation positions on the disaccharide backbone.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker W. Determination of antisera titres ing the single radial immunodiffusion method. Immunochemistry. 1969 Jul;6(4):539–546. doi: 10.1016/0019-2791(69)90193-1. [DOI] [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A., Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T., Morita T., Iwanaga S., Nakamura S., Niwa M. A new chromogenic substrate method for assay of bacterial endotoxins using Limulus hemocyte lysate. Prog Clin Biol Res. 1979;29:209–220. [PubMed] [Google Scholar]
- Inal S., Nagaki K., Ebisu S., Kato K., Kotani S. Activation of the alternative complement pathway by water-insoluble glucans of streptococcus mutans: the relation between their chemical structures and activating potencies. J Immunol. 1976 Oct;117(4):1256–1260. [PubMed] [Google Scholar]
- Kanegasaki S., Kojima Y., Matsuura M., Homma J. Y., Yamamoto A., Kumazawa Y., Tanamoto K., Yasuda T., Tsumita T., Imoto M. Biological activities of analogues of lipid A based chemically on the revised structural model. Comparison of mediator-inducing, immunomodulating and endotoxic activities. Eur J Biochem. 1984 Sep 3;143(2):237–242. doi: 10.1111/j.1432-1033.1984.tb08364.x. [DOI] [PubMed] [Google Scholar]
- Kotani S., Takada H., Tsujimoto M., Ogawa T., Harada K., Mori Y., Kawasaki A., Tanaka A., Nagao S., Tanaka S. Immunobiologically active lipid A analogs synthesized according to a revised structural model of natural lipid A. Infect Immun. 1984 Jul;45(1):293–296. doi: 10.1128/iai.45.1.293-296.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S., Takada H., Tsujimoto M., Ogawa T., Mori Y., Sakuta M., Kawasaki A., Inage M., Kusumoto S., Shiba T. Immunobiological activities of synthetic lipid A analogs and related compounds as compared with those of bacterial lipopolysaccharide, re-glycolipid, lipid A, and muramyl dipeptide. Infect Immun. 1983 Aug;41(2):758–773. doi: 10.1128/iai.41.2.758-773.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazawa Y., Matsuura M., Nakatsuru-Watanabe Y., Fukumoto M., Nishimura C., Homma J. Y., Inage M., Kusumoto S., Shiba T. Mitogenic and polyclonal B cell activation activities of synthetic lipid A analogues. Eur J Immunol. 1984 Feb;14(2):109–114. doi: 10.1002/eji.1830140202. [DOI] [PubMed] [Google Scholar]
- Lehmann V. Isolation, purification and properties of an intermediate in 3-deoxy-D-manno-octulosonic acid--lipid A biosynthesis. Eur J Biochem. 1977 May 2;75(1):257–266. doi: 10.1111/j.1432-1033.1977.tb11525.x. [DOI] [PubMed] [Google Scholar]
- Lüderitz O., Galanos C., Lehmann V., Mayer H., Rietschel E. T., Weckesser J. Chemical structure and biological activities of lipid A's from various bacterial families. Naturwissenschaften. 1978 Nov;65(11):578–585. doi: 10.1007/BF00364907. [DOI] [PubMed] [Google Scholar]
- Matsuura M., Kojima Y., Homma J. Y., Kubota Y., Shibukawa N., Shibata M., Inage M., Kusumoto S., Shiba T. Interferon-inducing, pyrogenic and proclotting enzyme of horseshoe crab activation activities of chemically synthesized lipid A analogues. Eur J Biochem. 1983 Dec 15;137(3):639–642. doi: 10.1111/j.1432-1033.1983.tb07873.x. [DOI] [PubMed] [Google Scholar]
- Obayashi T. Addition of perchloric acid to blood samples for colorimetric limulus test using chromogenic substrate: comparison with conventional procedures and clinical applications. J Lab Clin Med. 1984 Sep;104(3):321–330. [PubMed] [Google Scholar]
- Qureshi N., Takayama K., Heller D., Fenselau C. Position of ester groups in the lipid A backbone of lipopolysaccharides obtained from Salmonella typhimurium. J Biol Chem. 1983 Nov 10;258(21):12947–12951. [PubMed] [Google Scholar]
- Rosner M. R., Tang J., Barzilay I., Khorana H. G. Structure of the lipopolysaccharide from an Escherichia coli heptose-less mutant. I. Chemical degradations and identification of products. J Biol Chem. 1979 Jul 10;254(13):5906–5917. [PubMed] [Google Scholar]
- Rosner M. R., Verret R. C., Khorana H. G. The structure of lipopolysaccharide from an Escherichia coli heptose-less mutant. III. Two fatty acyl amidases from Dictyostelium discoideum and their action on lipopolysaccharide derivatives. J Biol Chem. 1979 Jul 10;254(13):5926–5933. [PubMed] [Google Scholar]
- Sidorczyk Z., Zähringer U., Rietschel E. T. Chemical structure of the lipid A component of the lipopolysaccharide from a Proteus mirabilis Re-mutant. Eur J Biochem. 1983 Dec 1;137(1-2):15–22. doi: 10.1111/j.1432-1033.1983.tb07789.x. [DOI] [PubMed] [Google Scholar]
- Stewart-Tull D. E., White R. G. The influence of age of culture on the production of adjuvant-active peptidoglycolipids by saprophytic mycobacteria. Immunology. 1967 Mar;12(3):349–359. [PMC free article] [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Mascagni P. Complete structure of lipid A obtained from the lipopolysaccharides of the heptoseless mutant of Salmonella typhimurium. J Biol Chem. 1983 Nov 10;258(21):12801–12803. [PubMed] [Google Scholar]
- Tanamoto K., Galanos C., Lüderitz O., Kusumoto S., Shiba T. Mitogenic activities of synthetic lipid A analogs and suppression of mitogenicity of lipid A. Infect Immun. 1984 May;44(2):427–433. doi: 10.1128/iai.44.2.427-433.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanamoto K., Zähringer U., McKenzie G. R., Galanos C., Rietschel E. T., Lüderitz O., Kusumoto S., Shiba T. Biological activities of synthetic lipid A analogs: pyrogenicity, lethal toxicity, anticomplement activity, and induction of gelation of Limulus amoebocyte lysate. Infect Immun. 1984 May;44(2):421–426. doi: 10.1128/iai.44.2.421-426.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal O., Jann K., Himmelspach K. Chemistry and immunochemistry of bacterial lipopolysaccharides as cell wall antigens and endotoxins. Prog Allergy. 1983;33:9–39. [PubMed] [Google Scholar]
- Yasuda T., Kanegasaki S., Tsumita T., Tadakuma T., Homma J. Y., Inage M., Kusumoto S., Shiba T. Biological activity of chemically synthesized analogues of lipid A. Demonstration of adjuvant effect in hapten-sensitized liposomal system. Eur J Biochem. 1982 May 17;124(2):405–407. [PubMed] [Google Scholar]
- Yasuda T., Kanegasaki S., Tsumita T., Tadakuma T., Ikewaki N., Homma J. Y., Inage M., Kusumoto S., Shiba T. Further study of biological activities of chemically synthesized analogues of lipid A in artificial membrane vesicles. Eur J Biochem. 1984 Apr 16;140(2):245–248. doi: 10.1111/j.1432-1033.1984.tb08094.x. [DOI] [PubMed] [Google Scholar]


