Abstract
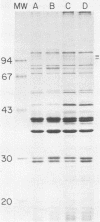
Haemophilus influenzae grown on enriched medium containing protoporphyrin IX rather than hemin was iron starved by the addition of the chelator ethylenediamine di-o-hydroxyphenylacetic acid. Iron starvation could be overcome in each of 33 H. influenzae type b isolates by 30% Fe-saturated human transferrin but not by human lactoferrin. Among nontypeable H. influenzae, 28 of 35 isolates, including 2 of 3 systemic isolates, were able to utilize Fe-transferrin. None of 18 H. parainfluenzae isolates was able to use Fe-transferrin. Iron starvation of H. influenzae type b resulted in increased amounts of three membrane proteins of 94,000 to 98,000 daltons.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIBERSTEIN E. L., MINI P. D., GILLS M. G. ACTION OF HAEMOPHILUS CULTURES ON DELTA-AMINOLEVULINIC ACID. J Bacteriol. 1963 Oct;86:814–819. doi: 10.1128/jb.86.4.814-819.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock J. H., Pickering M. G., McDowall M. C., Deacon A. G. Role of antibody and enterobactin in controlling growth of Escherichia coli in human milk and acquisition of lactoferrin- and transferrin-bound iron by Escherichia coli. Infect Immun. 1983 May;40(2):453–459. doi: 10.1128/iai.40.2.453-459.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton J. W., Brandt P., Mahoney J. R., Lee J. T., Jr Haptoglobin: a natural bacteriostat. Science. 1982 Feb 5;215(4533):691–693. doi: 10.1126/science.7036344. [DOI] [PubMed] [Google Scholar]
- Finch C. A., Huebers H. Perspectives in iron metabolism. N Engl J Med. 1982 Jun 24;306(25):1520–1528. doi: 10.1056/NEJM198206243062504. [DOI] [PubMed] [Google Scholar]
- Grahm G., Bates G. W. Approaches to the standardization of serum unsaturated iron-binding capacity. J Lab Clin Med. 1976 Sep;88(3):477–486. [PubMed] [Google Scholar]
- Helms S. D., Oliver J. D., Travis J. C. Role of heme compounds and haptoglobin in Vibrio vulnificus pathogenicity. Infect Immun. 1984 Aug;45(2):345–349. doi: 10.1128/iai.45.2.345-349.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochan I., Kvach J. T., Wiles T. I. Virulence-associated acquisition of iron in mammalian serum by Escherichia coli. J Infect Dis. 1977 Apr;135(4):623–632. doi: 10.1093/infdis/135.4.623. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F., Schonne E. Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J Exp Med. 1969 Sep 1;130(3):643–658. doi: 10.1084/jem.130.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickelsen P. A., Blackman E., Sparling P. F. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from lactoferrin. Infect Immun. 1982 Mar;35(3):915–920. doi: 10.1128/iai.35.3.915-920.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickelsen P. A., Sparling P. F. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from transferrin and iron compounds. Infect Immun. 1981 Aug;33(2):555–564. doi: 10.1128/iai.33.2.555-564.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles A. A., Khimji P. L. Enterobacterial chelators of iron: their occurrence, detection, and relation to pathogenicity. J Med Microbiol. 1975 Nov;8(4):477–490. doi: 10.1099/00222615-8-4-477. [DOI] [PubMed] [Google Scholar]
- Muller-Eberhard U. Hemopexin. N Engl J Med. 1970 Nov 12;283(20):1090–1094. doi: 10.1056/NEJM197011122832007. [DOI] [PubMed] [Google Scholar]
- Nagel R. L., Gibson Q. H. The binding of hemoglobin to haptoglobin and its relation to subunit dissociation of hemoglobin. J Biol Chem. 1971 Jan 10;246(1):69–73. [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- WHITE D. C., GRANICK S. HEMIN BIOSYNTHESIS IN HAEMOPHILUS. J Bacteriol. 1963 Apr;85:842–850. doi: 10.1128/jb.85.4.842-850.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalwijk C., van den Bosch J. F., MacLaren D. M., de Graaff J. Hemolysin plasmid coding for the virulence of a nephropathogenic Escherichia coli strain. Infect Immun. 1982 Jan;35(1):32–37. doi: 10.1128/iai.35.1.32-37.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. H., Warner P. J. ColV plasmid-mediated, colicin V-independent iron uptake system of invasive strains of Escherichia coli. Infect Immun. 1980 Aug;29(2):411–416. doi: 10.1128/iai.29.2.411-416.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]