Abstract
Expression of the CD45 Ag in hemopoietic cells is essential for normal development and function of lymphocytes, and both mice and humans lacking expression exhibit SCID. Human genetic variants of CD45, the exon 4 C77G and exon 6 A138G alleles, which alter the pattern of CD45 isoform expression, are associated with autoimmune and infectious diseases. We constructed transgenic mice expressing either an altered level or combination of CD45 isoforms. We show that the total level of CD45 expressed is crucial for normal TCR signaling, lymphocyte proliferation, and cytokine production. Most importantly, transgenic lines with a normal level, but altered combinations of CD45 isoforms, CD45RABC/+ and CD45RO/+ mice, which mimic variant CD45 expression in C77G and A138G humans, show more rapid onset and increased severity of experimental autoimmune encephalomyelitis. CD45RO/+ cells produce more TNF-α and IFN-γ. Thus, for the first time, we have shown experimentally that it is the combination of CD45 isoforms that affects immune function and disease.
The CD45 (leukocyte common) Ag is a hemopoietic cell-specific tyrosine phosphatase, essential for efficient T and B cell Ag receptor signal transduction (1, 2). Multiple CD45 isoforms can be generated by complex alternative splicing of exons 4(A), 5(B), and 6(C) in the extracellular domain of the molecule. The expression of different CD45 isoforms is cell type specific and depends on the state of activation and differentiation of hemopoietic cells (3, 4). In humans, naive T lymphocytes express high molecular mass isoforms containing the A exon (CD45RA cells), but, following activation, the low molecular mass 180-kDa isoform is expressed (CD45RO cells), and CD45RO expression is maintained on the majority of primed (memory) cells (3, 5). In both rats and mice, low molecular mass isoform expression is also associated with T cell memory and more differentiated T cell function (4, 6).
Although CD45 alternative splicing is highly regulated and conserved among vertebrates, the function of different CD45 isoforms is not clear. No specific ligand has been found for CD45, although interactions with lectin-like molecules have been reported (7-10). Nevertheless, it is clear that a large CD45 extracellular domain is required for normal TCR signaling, at least in transfected cell lines (11). The formation of homo- and heterodimers by CD45 isoforms has been proposed as another mechanism that may regulate CD45 phosphatase activity (12); however, recent structural data indicate that this is unlikely (13). Spatiotemporal mechanisms for regulating CD45 activity have been suggested involving partitioning of CD45 into lipid rafts or the immunological synapse (14-17).
However, although the function of CD45 isoforms remains unknown, what is clear is that CD45 is crucial for normal immune function and altered CD45 expression has major effects. Both mice and humans lacking CD45 expression are severely immunodeficient (18, 19), and point mutations of CD45 that cause altered splicing have been associated with autoimmune and infectious diseases (20-22). The two best described human CD45 variant alleles have contrasting phenotypes, geographical distribution, and disease associations. The exon 4 C77G variant is found in 1–3% of Europeans and North Americans and is present at a higher frequency in multiple sclerosis (MS),2 HIV, autoimmune hepatitis, systemic sclerosis, and other diseases (20, 22-25). C77G carriers cannot splice out exon A, and their memory/effector lymphocytes continue to express both CD45RA and CD45RO isoforms. In contrast, exon 6 A138G is present in nearly 40% of Far Eastern Orientals (26). The allele promotes splicing toward the CD45RO isoform, resulting in the presence of more memory/effector CD45RO-positive T cells and more T cells producing IFN-γ. A138G is associated with protection against Graves' disease and hepatitis B (21). The high frequency of A138G indicates that it may also affect susceptibility and pathogenesis in other autoimmune or infectious diseases, and therefore, has an important effect on disease burden in a large part of the human population.
At least two mechanisms may underlie CD45 regulation of immune function. The first is by control of the threshold of signaling through the TCR. The critical role of CD45 phosphatase activity for TCR signal transduction is well established, and the Src family of kinases has been identified as its primary targets (1, 2). The second mechanism is by regulation of cytokine production and responses. CD45−/− mice have increased phosphorylation of Jaks, and their hemopoietic cells show increased responses to cytokines (27), while recent studies have shown that CD45 is required for chemokine and cytokine production in NK cells after stimulation via Fc or MHC-binding receptors (28).
To investigate the role of distinct CD45 isoforms, we constructed transgenic (Tg) mice expressing single high (CD45RABC) or low (CD45RO) molecular mass isoforms at different levels on a CD45−/− background. Because in humans expressing CD45 variant alleles it is the combinations of isoforms expressed on cells that are altered, we also generated mice expressing defined combinations of isoforms mimicking the expression of C77G or A138G human variants. In these CD45 Tg models, we asked how CD45 expression patterns affect proliferative responses and cytokine production. We show first that a threshold of CD45 expression is required for adequate TCR signaling and cytokine production. Second, we show that changes in combinations of CD45 isoforms alter the threshold for TCR signaling and cytokine production, resulting in altered susceptibility and severity of disease in a model autoimmune disease, experimental autoimmune encephalomyelitis (EAE). Collectively, our results show that both the levels of expression and combinations of CD45 isoforms are crucial for lymphocyte function.
Materials and Methods
CD45 Tg mice
Tg mice expressing single CD45RABC or CD45RO isoforms under the control of the human CD2 promoter have been described previously (29). The Tg mice were bred onto CD45 homozygous knockout mice. Two lines with each isoform were constructed with high and low levels of transgene expression. These are referred to as CD45RABChigh, CD45RABClow, CD45ROhigh, and CD45ROlow. CD45 Tg mice expressing two isoforms (CD45RABC × CD45RO) were generated by breeding CD45RABChigh and CD45ROhigh mice. We also generated mice with one normally splicing CD45 allele and a fixed nonsplicing single CD45 transgene (CD45RABC/+ and CD45RO/+ mice) by breeding single isoform CD45RABChigh or CD45ROhigh Tg mice with C57BL/6 mice. The presence of the CD45 transgenes in the F1 crosses was detected by PCR on tail genomic DNA, using forward, 5′-GAGCTCAGAATCAAAAGAGGA-3′ and reverse, 5′-TAATTCACAGTAATGTTCCCAAACATGGC-3′ primers, generating 1000- and 710-bp products for the CD45RABC and CD45RO transgenes, respectively. All mice were bred in the specific pathogen-free facilities of the Institute for Animal Health and used for experiments at 6–8 wk of age, unless otherwise stated. All experiments fully complied with relevant Home Office guidelines and were approved by the animal ethical committee of the Institute for Animal Health.
Flow cytometric analysis
The following reagents and Abs were also used to stain cell suspensions: CD4 FITC (GK1.5), CD8 PE (53-6.7), pan CD45 CyC (30-F11), CD45RA biotin (14.8), and CD45RB biotin (16A), all from BD Biosciences. Apoptosis was visualized by annexin V staining. Cells were first surface stained using CD4 and CD8 Abs, followed by annexin staining, according to the manufacturer's instruction (BD Pharmingen).
T cell separation and activation
CD4 and CD8 lymph node T cells were purified by negative selection by first incubating the cells with anti-MHC class II (clone TIB120), B220 (clone RA36B2), CD11b (clone M1/70), and either CD4 (GK1.5) or CD8 (3168) Abs (all gifts from D. Tough, The Edward Jenner Institute for Vaccine Research, Compton, U.K.), followed by Dynabead separation. The purity of the resulting subsets was checked by flow cytometry and was better than 90%.
For T cell stimulation, 2 × 105 lymph node cells were placed into round-bottom 96-well plates. PMA (50 ng/ml) and calcium ionophore (ionomycin; 200 ng/ml) were added to the wells. For TCR-CD3 cross-linking, the plates were coated overnight at 4°C with varying concentrations (from 0 to 10 μg/well) of anti-mouse CD3∈ (clone 145-2C11; BD Biosciences) in the presence or absence of 1 μg/well CD28 (clone 37.51; BD Biosciences) and washed three times before incubation with the T cells. For MLR, 2 × 105 lymph node cells of CD45 Tg H-2b mice were placed into round-bottom 96-well plates in the presence of 2 × 106 (10:1), 1 × 106 (5:1), and 2 × 105 (1:1) T cell-depleted BALB/c H-2d stimulator cells. These were prepared by treating the splenocytes with anti-Thy-1.2 mAb, followed by addition of guinea pig complement (gifts from D. Tough) and irradiating with 2500 rad. T cells were harvested at various intervals (24–96 h) after a 12-h pulse with 1 μCi of [3H]thymidine per well.
Cytokine production
Cytokine production was measured using BD Biosciences mouse Th1/Th2 cytokine cytometric bead array kit, following the manufacturer's protocol. Briefly, for each test sample, 10 μl of each cytokine capture bead suspension was mixed, and 50 μl of the mixed beads was transferred to each assay tube, followed by 50 μl of PE detection reagent and 50 μl of diluted standard or neat sample. The samples were incubated for 2 h at room temperature in the dark, washed, and resuspended in 300 μl of wash buffer. The standards and test samples were analyzed on a FACSCalibur (BD Biosciences), using CellQuest and BD cytometric bead array software, in accordance with the manufacturer's instructions.
Western blot analysis
For total protein extraction, cell lysis (2 × 106 cells/ml) was performed in radioimmunoprecipitation assay buffer (PBS (pH 7.4), 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS,10 mM NaF, and 0.5 mM PMSF) buffer, containing protease and phosphatase inhibitors (protease inhibitor mixture and phosphatase inhibitor mixture 2; Sigma-Aldrich), on ice at 4°C for 30 min. Insoluble materials were removed by centrifugation (15,000 × g for 10 min at 4°C), and the total protein concentration of each sample was quantified by bicinchoninic acid protein assay (Pierce Biotechnology). Cell lysate proteins were analyzed by 10% SDS-PAGE (10 μg of total protein per lane), transferred onto a nitrocellulose membrane, and immunoblotted using Abs specific for STAT1, pY701STAT1 (Upstate Biotechnology), and T-bet (Santa Cruz Biotechnology). The basal and phosphorylated status of Lck was determined using Abs against Lck (Upstate Biotechnology), pY505Lck, or pY416Src (Cell Signaling Technology). pY416Src Ab cross-reacts with pY394Lck. Blots were developed using ECL donkey anti-rabbit HRP-linked F(ab′)2 and ECL Western blotting detection reagents (Amersham Biosciences).
Induction of EAE
EAE was induced by s.c. injection of myelin oligodendrocyte glycoprotein peptide35–55 (MEVGWYRSPFSRVVHLYRNGK) consisting of 200 μg of peptide Ag in a final volume of 100 μl of CFA (Difco Laboratories) containing Mycobacterium tuberculosis (4 mg/ml) (30). On the same day and 2 days later, all mice received two i.p. doses of pertussis toxin (200 ng/injection; Sigma-Aldrich). Mice were scored twice daily for symptoms of EAE, as follows: 0, no signs; 1, flaccid tail; 2, partial hind limb paralysis and/or impaired righting reflex; 3, full hind paralysis; 4, hind limb plus fore limbs paralysis; and 5, moribund and dead. The two scores for each day were added together and divided by the number of surviving mice to give the mean EAE grade for each day.
Statistical analysis
Student's t test assuming equal variance was used.
Results
Proliferative responses in CD45 Tg mice expressing single CD45RABC or CD45RO isoforms
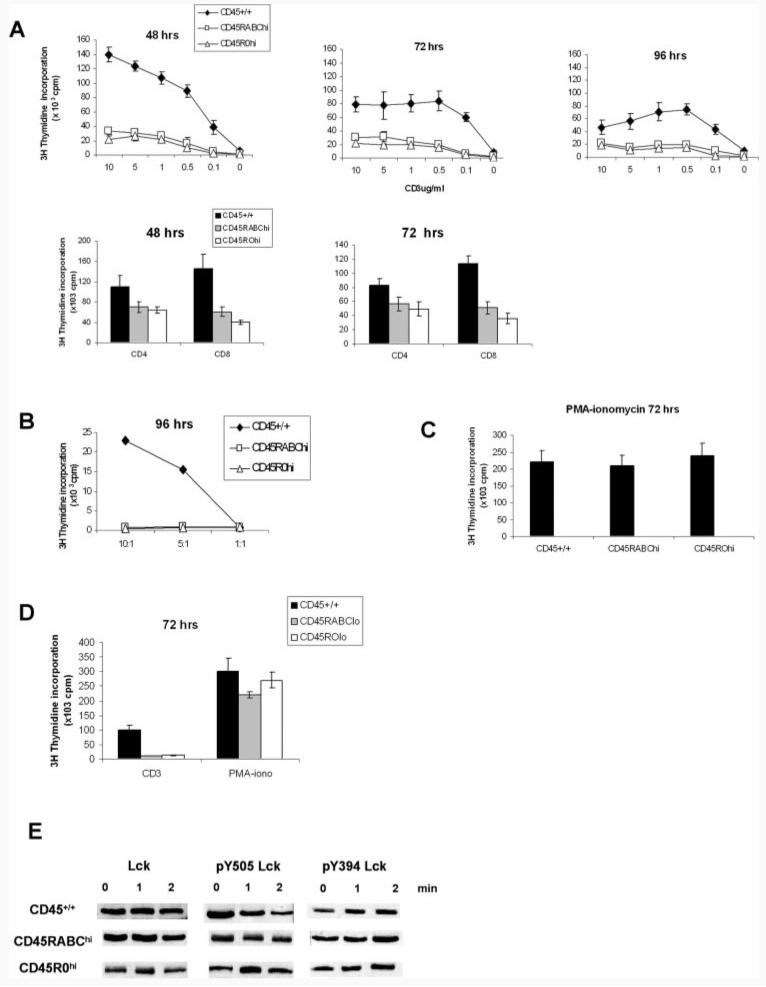
To investigate the role of different CD45 isoforms in T cell function, we constructed Tg mice expressing single high (CD45RABC) or low (CD45RO) molecular mass isoforms on a CD45−/− background (29). Two CD45RABC and two CD45RO lines were generated with high and low levels of expression of the respective transgenes (Fig. 1). These are referred to as CD45RABChigh, CD45RABClow, CD45ROhigh, and CD45ROlow. CD3+ cells from CD45RABChigh and CD45ROhigh lines showed comparable levels of cell surface expression, while the CD45RABClow had a higher level of expression compared with the CD45ROlow mice (Fig. 1). Cell surface expression of CD45 on T cells from the CD45RABChigh and CD45ROhigh was ∼5-fold lower than the level of total CD45 in wild-type CD45+/+ mice.
FIGURE 1.
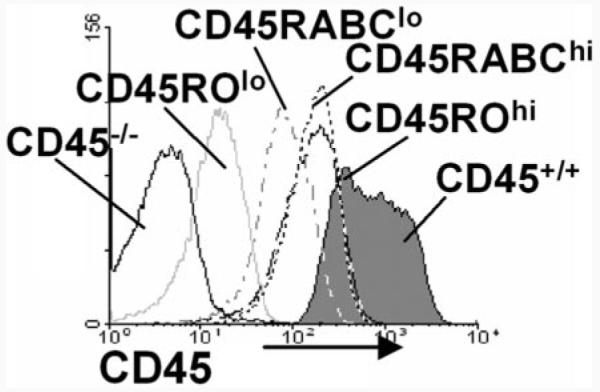
Characterization of single isoform CD45RABC and CD45RO Tg mice. Flow cytometric analysis showing the surface expression of CD45, detected with a pan-specific anti-CD45 mAb, on CD3-gated thymocytes from CD45+/+ (filled histogram), CD45−/−, CD45RABChigh, CD45RABClow, CD45R0high, and CD45R0low mice.
We compared the responses to titrated amounts of plate-bound CD3/CD28 over time, of Tg or control CD45+/+ lymph node T cells. Proliferation of both CD45RABChigh and CD45ROhigh cells was greatly reduced compared with CD45+/+ cells throughout the response (Fig. 2A). Even in the presence of IL-2, although the response was increased, it was still no more than 50% of that of CD45+/+ mice (data not shown). The number of T cells in lymph nodes in CD45RABChigh and CD45ROhigh was the same as in CD45+/+ mice, as detected by CD3, CD4, and CD8 FACS analysis, and did not account for the reduced response. Both CD4 and CD8 T cells showed similarly reduced responses (Fig. 2A). Neither CD45RABChigh nor CD45ROhigh cells were able to respond to alloantigens in MLR (Fig. 2B), while both CD45RABChigh and CD45ROhigh lymph node T cells responded to PMA-ionomycin as well as wild-type CD45+/+ cells (Fig. 2C). No difference between the proliferative responses of CD45RABChigh or CD45ROhigh cells to any of the stimuli was detected.
FIGURE 2.
Activation of lymph node T cells from CD45+/+ and CD45 Tg mice expressing single isoforms. A, Total mesenteric and peripheral lymph node cells from CD45RABChigh and CD45ROhigh mice were stimulated with varying amounts of plate-bound CD3 and 1 μg/ml CD28 Abs. Separated CD4 and CD8 lymph node cells were stimulated with 2 μg/ml CD3 and 1 μg/ml CD28 Abs. B, Lymph node cells were stimulated with T cell-depleted and irradiated allogeneic BALB/c splenocytes at ratios of 10:1, 5:1, and 1:1 (stimulator:target), or C, with PMA-ionomycin. D, CD45RABClow and CD45ROlow lymph node cells were stimulated with 2 μg/ml plate-bound CD3 and 1 μg/ml CD28 Abs or with PMA-ionomycin. Cells were pulsed with [3H]thymidine at the indicated times and harvested 12 h later. Means and SDs of triplicate cultures from three mice are shown. Background counts were <500 cpm and have been subtracted. E, Western blot of Lck, pY505Lck, and pY394Lck in lymph node T cells stimulated with soluble CD3 at 2 μg/ml and CD28 at 1 μg/ml for the times indicated. Equal amount of cell lysate protein (10 μg) was loaded on each lane. Data are representative of six experiments.
In contrast with CD45RABChigh and CD45ROhigh, mice of the low expressing lines (CD45RABClow and CD45ROlow) gave minimal responses to CD3/CD28, while the response to PMA and ionomycin was not affected (Fig. 2D), suggesting that the level of CD45 expressed influences the ability of T cells to respond to TCR stimulation and that a threshold level of CD45 single isoform expression is required, below which no response can be generated.
To understand the basis for the reduced response of CD45RABChigh and CD45ROhigh, we next examined one of the early phosphorylation events downstream of the TCR. Lck is a substrate for CD45 phosphatase activity, and the dominant role of CD45 is to dephosphorylate the inhibitory tyrosine Y505Lck. Therefore, we measured the basal level of Lck and its pY505Lck phosphorylation status following activation via CD3/CD28. As shown in Fig. 2E, the level of Lck protein is the same in the control CD45+/+, CD45RBAChigh, and CD45ROhigh mice. However, pY505Lck is reduced at 2 min in CD45+/+, but not in the CD45RABChigh and CD45ROhigh cells. These results show that there is reduced phosphatase activity in cells expressing CD45RABChigh and CD45ROhigh single isoforms, which explains the decreased proliferative response to CD3/CD28 and suggests that a higher level of expression or a combination of isoforms is required to restore this defect. Because CD45 can also dephosphorylate the activating tyrosine Y394Lck, we also measured its phosphorylation using Ab against Src phosphorylated at tyrosine 416. No differences in phosphorylation of Y394Lck were detected between the three mice strains (Fig. 2E).
Taken together, these results support two important conclusions. First, a threshold level of single CD45 isoform expression is required for TCR signaling. Second, cells expressing single CD45RABC or CD45RO isoforms at similar levels show identical patterns of response.
Cytokine production in CD45 Tg mice expressing single CD45RABC or CD45RO isoforms
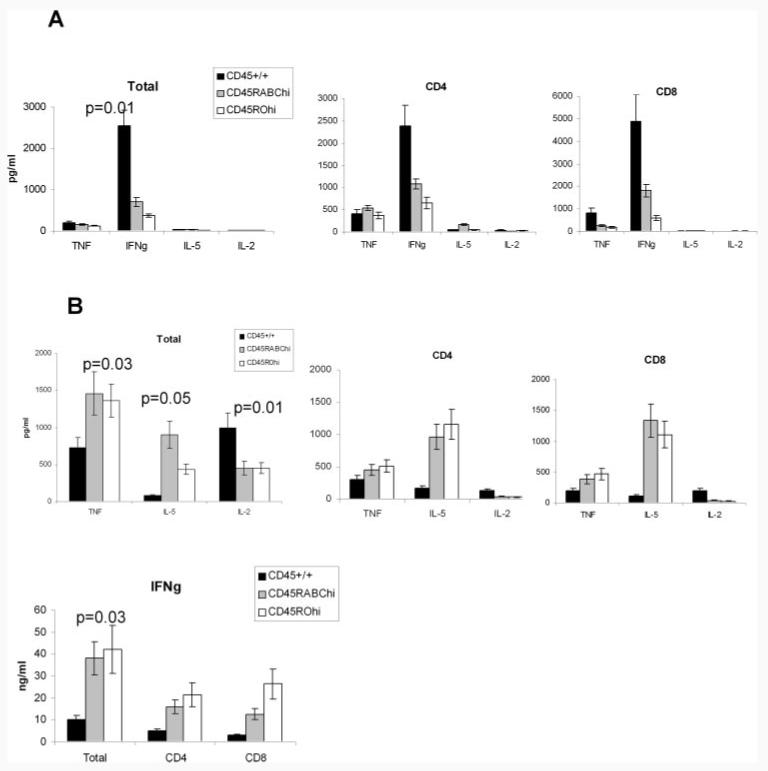
We next studied the role of single CD45RABC and CD45RO isoforms in cytokine production. There were reduced amounts of IFN-γ in the supernatants of CD45 Tg cells compared with controls (Fig. 3A), while no consistent changes in TNF production were seen. IL-5 and IL-2 were not detected in supernatants. When separated CD4 and CD8 cells were analyzed, IFN-γ was reduced in both subsets.
FIGURE 3.
Cytokine production by total or CD4- and CD8-separated lymph node cells of CD45 Tg mice expressing single CD45RABC or CD45RO isoforms. A, Amount of TNF-α, IFN-γ, IL-5, and IL-2 present in cell culture supernatants after 72-h stimulation with 2 μg/ml CD3 and 1 μg/ml CD28, or B, PMA-ionomycin. Histograms show the response and SD of three mice of each type. Data are representative of four experiments for total CD3-positive cells and two experiments for CD4- and CD8-separated cells. Statistically significant differences from wild-type CD45+/+ controls are shown.
We also measured cytokine production after stimulation of lymph node cells with PMA and ionomycin. PMA-ionomycin bypasses CD3 signaling, causing Ca2+ influx directly and activation of PKC. Increased production of TNF-α, IL-5, and IFN-γ and decreased IL-2 were detected in both CD45 Tg mice at 72 h (Fig. 3B) as well as at 24 and 48 h (data not shown). Similar trends were observed in supernatants of separated CD4 and CD8 cells. Increased cytokine production was detected also in the low expressing CD45RABClow and CD45ROlow mice (data not shown). No significant differences were observed between the CD45 Tg mice irrespective of the level or isoform expressed, suggesting that these cells do have the capacity both to proliferate and produce cytokines if TCR signaling is bypassed.
At first sight, the differences in cytokine production of single isoform Tg cells following stimulation with CD3/CD28 or PMA-ionomycin are puzzling. However, single isoform mice have mostly activated/effector type T cells (29). When stimulated with CD3/CD28, the threshold of TCR signaling is not reached so that less cytokines are produced than by CD45+/+ control cells. In contrast, when PMA-ionomycin bypasses TCR signaling, abundant effector cytokines are produced by the activated effector T cells in the Tg mice. Less IL-2 is detected because this cytokine is produced by naive T cells.
These data suggest that, as with the proliferative response, a higher level of CD45 expression or more than one CD45 isoform is required for normal cytokine production.
Responses in mice expressing two CD45 isoforms
Mice expressing single CD45RABChigh or CD45ROhigh at levels five times less than the total CD45 in wild-type CD45+/+ mice give grossly abnormal in vitro proliferative and cytokine responses. These results, therefore, indicate that a higher level of single CD45 isoform expression or, alternatively, a combination of isoforms is required to reconstitute normal proliferative and cytokine responses. We next asked whether combinations of CD45 isoforms would restore these responses, first studying the response of T cells in mice expressing both CD45RABC and CD45RO isoforms (CD45RABChigh × CD45ROhigh). Neither the proliferative nor cytokine responses were reconstituted by the simultaneous expression of CD45RABC and CD45RO isoforms, and the responses were not different from single CD45RABChigh or CD45ROhigh cells (data not shown). However, although twice as much CD45 was expressed on the surface of CD45RABChigh × CD45ROhigh cells, the total level of CD45 is still less than in CD45+/+ mice. Therefore, it appears that the threshold level for normal responses was not reached or that more than two isoforms are required to restore the response.
Proliferative and cytokine responses in CD45 Tg/+ mice
Because in humans expressing C77G or A138G CD45 variant alleles it is the combinations of isoforms expressed on cells that are altered rather than the total amount of CD45, we generated mice expressing one normally splicing CD45 allele and a CD45RABC or CD45RO transgene. We constructed CD45RABC/+ mice (F1 of CD45RABChigh × CD45+/+), which mimic the expression in C77G variant humans, and CD45RO/+ mice (F1 of CD45ROhigh × CD45+/+), which model the isoform expression of the A138G allele.
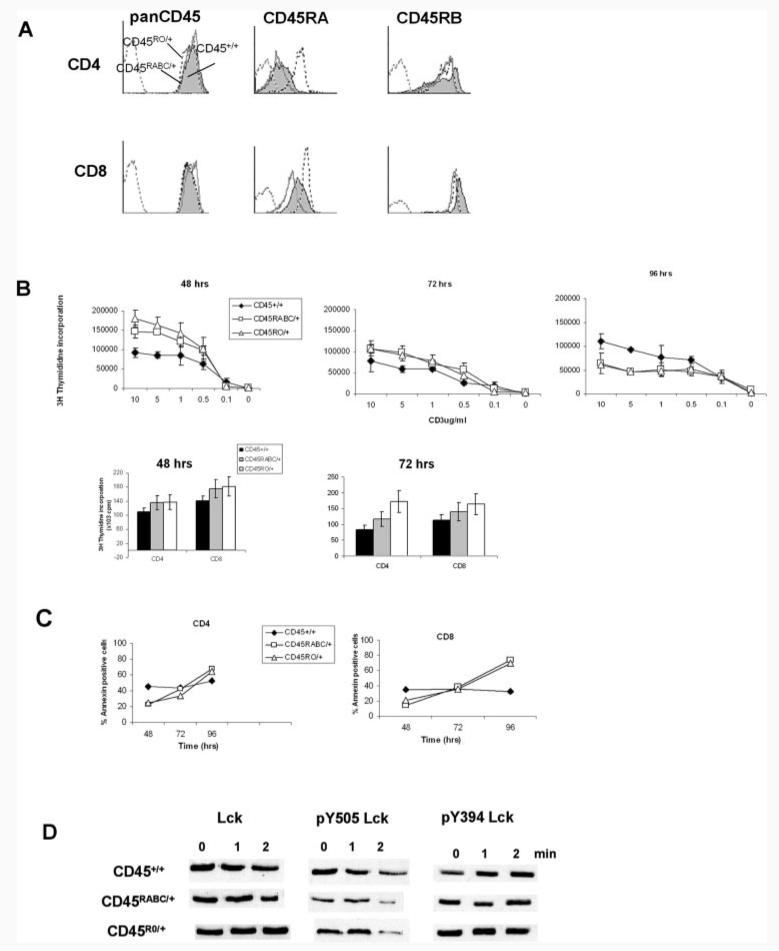
We analyzed the expression of CD45 isoforms and total CD45 expression in CD4 and CD8 cells (Fig. 4A). The level of total CD45 expression is very similar in both CD4 and CD8 cells in CD45+/+, CD45RABC/+, and CD45RO/+ mice. However, staining with CD45RA indicates that the balance of different isoforms is altered in these mice. As expected, more CD45RA is detected in CD45RABC/+ cells because of the presence of the CD45RABC transgene. Both CD45RABC/+ and CD45RO/+ cells have a lower expression of CD45RB isoforms in CD4 and CD8 cells, suggesting more splicing of the normal CD45 allele toward lower molecular mass isoforms. Both Tg strains have up to 20% more activated T cells, as detected by CD44 and CD122 staining (data not shown), which may also explain the lower CD45RB expression seen in both lines.
FIGURE 4.
Characterization of CD45RABC/+ and CD45RO/+ mice. A, CD45 expression in CD45RABC/+ and CD45RO/+ mice. Lymph node cells were stained with pan CD45, CD45RA, and CD45RB isoform-specific Abs. Analysis was performed on CD4- and CD8-gated cells. The shaded histogram is CD45+/+, dotted line CD45RABC/+, solid CD45RO/+, and the gray dotted isotype control. Examples are representative of three mice of each type. B, Activation of T cells from CD45+/+, CD45RABC/+, and CD45RO/+ mice. Mesenteric and peripheral lymph node cells were activated in the presence of varying amounts of plate-bound CD3 and 1 μg/ml CD28 Abs. Separated CD4 and CD8 lymph node cells were stimulated with 2 μg/ml CD3 and 1 μg/ml CD28 Abs. Cells were pulsed with [3H]thymidine at the indicated times and harvested after 12 h. Means and SDs of triplicate cultures from three mice are shown. Background counts were <500 cpm and have been subtracted. Data are representative of five experiments. C, Annexin staining of cells cultured in the presence of 2 μg/ml CD3 and 1 μg/ml CD28 for the indicated times. Means and SDs of triplicate cultures from three mice are shown. D, Western blot of Lck, pY505Lck, and pY394Lck in lymph node T cells stimulated with 2 μg/ml CD3 and 1 μg/ml CD28 for the times indicated. Data are representative of three experiments.
Because the total level of CD45 expression is normal, we next determined whether the alterations in the balance of isoforms would affect CD3/CD28 responses. Lymphocytes from lymph nodes of CD45+/+, CD45RABC/+, or CD45RO/+ mice were activated with plate-bound CD3/CD28. We observed differences between the response of the wild-type CD45+/+ and the CD45RABC/+ and CD45RO/+ mice. In the first 48 h, CD45RABC/+ and CD45RO/+ cells show clearly increased proliferation, followed by a reduction at 96 h compared with CD45+/+ mice (Fig. 4B). Both CD4 and CD8 responses were affected. Because proliferative responses are a balance of cell division and cell death, we studied apoptosis during responses to CD3/CD28. Fig. 4C shows that the pattern of apoptosis is a mirror image of the proliferative responses, with the CD45RABC/+ and CD45RO/+ mice showing less apoptosis at 48 h, but an increase compared with CD45+/+ control cells at 96 h.
We assessed the levels of Lck and pY505Lck phosphorylation. Basal levels of Lck are the same in the three strains of mice, but pY505LCK in CD45RABC/+ and CD45RO/+ cells shows greater dephosphorylation at 2 min following CD3/CD28 stimulation (Fig. 4D). The increased dephosphorylation correlates with the brisk initial proliferative response and suggests increased CD45 phosphatase activity in the CD45RABC/+ and CD45RO/+ mice. No changes in the phosphorylation pattern of pY394Lck were detected.
These results indicate clearly that the altered balance of isoforms in both CD45RABC/+ and CD45RO/+ mice does lead to altered magnitude and kinetics of proliferative responses compared with CD45+/+ mice. However, no differences between the proliferative responses of CD45RABC/+ and CD45RO/+ cells were detected.
Cytokine production in CD45 Tg/+ mice
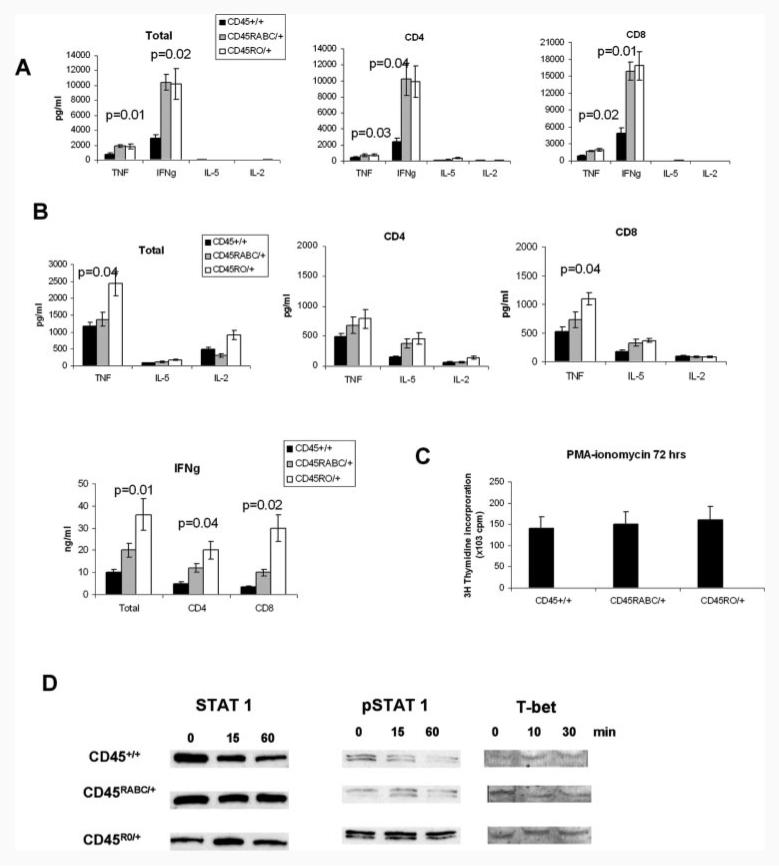
We next analyzed cytokine production in CD45RABC/+ and CD45RO/+ mice after stimulation with CD3/CD28 or PMA-ionomycin. Both CD45RABC/+ and CD45RO/+ cells produced more TNF-α and IFN-γ compared with CD45+/+ in response to CD3/CD28 stimulation, although there were no differences between the two Tg mice (Fig. 5A). Similar trends in cytokine production were seen in supernatants of separated CD4 and CD8 lymphocytes.
FIGURE 5.
Cytokine production and response of CD45+/+, CD45RABC/+, and CD45RO/+ mice. A, Total lymph node cells or separated CD4 and CD8 cells were stimulated for 72 h with CD3/CD28, or B, PMA-ionomycin. The histograms show means and SD of the amounts in supernatants of triplicate cultures from three mice. Results are representative of six experiments for total lymph node cells and three for separated CD4 or CD8 cells. C, Activation of lymph node T cells with PMA-ionomycin. Cells were pulsed with [3H]thymidine at 72 h and harvested 12 h later. Means and SDs of triplicate cultures from three mice are shown. D, Western blot of STAT1, pY701STAT1, and T-bet in lymph node T cells stimulated with PMA-ionomycin for the times indicated. Data are representative of three experiments.
Interestingly, when stimulated with PMA-ionomycin, the CD45RO/+ mice consistently showed the highest production of TNF-α and IFN-γ at 72 h (Fig. 5B). Increased TNF and IFN-γ production was also observed in separated CD4 and CD8 subsets. There was less consistent trend to production of more IL-2 and IL-5 in CD45RABC/+ and CD45RO/+ mice. No IL-4 was detected in the supernatants. Despite finding differences in cytokine production, proliferative responses to PMA-ionomycin are the same in all strains of mice (Fig. 5C).
Because the change in IFN-γ production was particularly striking and because increased IFN-γ was detected in the human A138G variant individuals (21), for which the CD45RO/+ mice are a model, we concentrated on understanding the increased production of IFN-γ in CD45RO/+ cells. It has been shown that PMA-ionomycin-induced IFN-γ production is dependent on STAT1 and that the Th1-specific transcription factor T-bet, which increases IFN-γ production, is regulated by IFN-γ signaling through STAT1 (31). We therefore measured the basal and phosphorylated level of STAT1 and T-bet protein following PMA-ionomycin stimulation. Fig. 5D indicates that in CD45RO/+ mice more phosphorylated STAT1 and more T-bet are detected, providing an explanation for the increased IFN-γ production in these mice.
EAE in CD45 Tg mice
Human CD45 variant alleles have been associated with autoimmune diseases. The C77G variant has been found with increased frequency in MS (20, 25, 32), while the A138G allele has been shown to be protective in Graves' disease (21). Because the CD45RABC/+ and CD45RO/+ mice have altered TCR signaling and cytokine production, we next tested whether this would lead to altered disease susceptibility or progression. EAE is a model for both MS in humans and the induction of autoimmune reactivity. We therefore used a myelin oligodendrocyte glycoprotein peptide to induce EAE in CD45+/+, CD45RABC/+, and CD45RO/+ mice (30).
Both CD45RABC/+ and CD45RO/+ developed more severe disease compared with CD45+/+ mice with average total score of 2.2 ± 0.8 and 2.5 ± 1.1, respectively, vs 1.6 ± 1.1 in control CD45+/+ mice (Table and Fig. 6). Disease onset was also earlier in CD45RABC/+ and CD45RO/+ mice (day 9.6 ± 1.1 and 10.3 ± 1.8 vs 12.6 ± 2.4 in CD45+/+ mice). In all three experiments, the CD45RABC/+ mice developed the disease first (day 9.6 ± 1.1), while the signs of disease were more severe in CD45RO/+ mice (average maximal disease score 4.8 ± 1.3 vs 3.5 ± 0.9 and 3.1 ± 1.1).
Table I.
EAE induction and assessment in CD45+/+, CD45RABC/+, and CD45RO/+ micea
| Incidence of Disease |
Mean Day of Onset (±SD) |
Average Total Score (±SD) |
Average Maximal Score (±SD) |
Fatality | |
|---|---|---|---|---|---|
| CD45+/+ | 21/22 | 12.6 ± 2.4 | 1.6 ± 1.1 | 3.1 ± 1.1 | 7/22 |
| CD45RABC/+ | 24/25 | 9.6 ± 1.1* | 2.2 ± 0.8*** | 3.5 ± 0.9 | 6/25 |
| CD45RO/+ | 19/20 | 10.3 ± 1.8* | 2.5 ± 1.1*** | 4.8 ± 1.3** | 9/20 |
EAE was induced in groups of five to nine mice. The mean and SDs from three experiments are shown. The average total score was calculated by adding the scores from each day from the onset of disease and dividing by the number of days scored. The average maximal score was the maximal score in each experiment for each strain of mice divided by three. Statistically significant differences from wild-type CD45+/+ controls are indicated as
p < 0.05
p < 0.01
p < 0.001.
FIGURE 6.
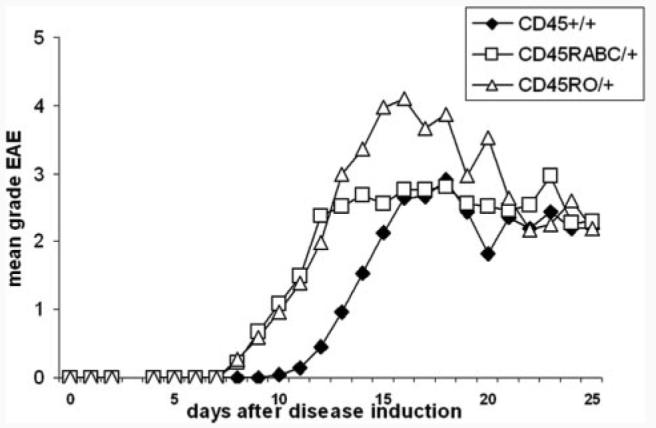
EAE in CD45+/+, CD45RABC/+, and CD45RO/+ mice. EAE was induced in groups of five to nine mice of each strain, and disease was monitored, as described in Materials and Methods. The graph represents the mean score for each day from three experiments.
These results indicate that altering the balance of isoforms is important for immune function, and that CD45RABC/+ and CD45RO/+ mice are more susceptible to induction of autoimmune disease in the EAE model.
Discussion
CD45 expression patterns can have profound effects on immune function and disease, and CD45 variant alleles that alter CD45 isoform expression are associated with infectious and autoimmune diseases. However, how CD45 isoform expression regulates lymphocyte function is not clear. To understand this, we studied how TCR signaling, cytokine production, and disease are altered in Tg mice expressing single, combinations of two, or more complex combinations of CD45 isoforms.
Our results with Tg mice expressing single CD45RABChigh, CD45ROhigh, or CD45RABClow and CD45ROlow isoforms show that the level of CD45 expression is critical for normal TCR signaling and induction of proliferative and cytokine responses. This threshold has not been reached in our single CD45RABChigh and CD45ROhigh Tg mice because they mount suboptimal in vitro proliferative and cytokine responses. We suggest that the suboptimal response in single isoform Tg mice is most likely due to the level of expression rather than the lack of a combination of isoforms for three reasons. First, mice with higher level of a single CD45 isoform than ours give normal proliferative responses (33). Second, the magnitude of proliferative response correlates with the level of expression in our single isoform high and low CD45RABC and CD45RO mice. Third, combinations of two isoforms at a subnormal level do not restore the response.
Interestingly, both CD45RABChigh and CD45ROhigh mice are able to mount protective immune response during lymphocytic choriomeningitis virus and influenza virus infection (29), suggesting that in the context of a viral infection, when the innate system is strongly activated to produce cytokines and coreceptor signals (34), the activation threshold may be reached, allowing productive signaling to occur. However, when less innate activation occurs, a threshold level for an adequate response is not achieved. In addition to showing the importance of adequate levels of CD45 expression, both the in vitro and in vivo data indicate that there is no difference in the proliferative response and cytokine production between cells expressing either single CD45RABC or CD45RO isoforms at the same level or a combination of these two.
All hemopoietic cells express more than one CD45 isoform, and alternative splicing is conserved throughout vertebrate evolution, so that it seems certain that combinations of isoforms must have vital functions. Therefore, the most important new finding reported in this study is that combinations of CD45 isoforms do indeed affect immune function. Thus, even when a normal level of total CD45 expression was reached, but the balance of isoforms changed, as in CD45RABC/+ or CD45RO/+ mice, both strains showed altered kinetics and magnitude of proliferative response accompanied by more rapid and efficient dephosphorylation of pY505Lck. This leads to an altered TCR threshold reflected in the rapid proliferative responses of CD45RABC/+ or CD45RO/+ cells. As a result of the more vigorous TCR response, more TNF-α and IFN-γ are produced by CD45RABC/+ or CD45RO/+ cells.
Convincing differences in the function of distinct CD45 isoforms have been difficult to identify. Many studies have been performed in which specific CD45 isoforms have been transfected into transformed cell lines. In some cases, a CD45RO transgene, while in others CD45ABC, was more efficient in restoring TCR signaling (35-38). These inconsistent observations are most likely due to inadequate levels of CD45 isoforms expressed, differences in CD45 negative cell lines, and in the stimuli or assays used (1). In contrast, our studies and those of others (33) with CD45 Tg mice expressing identical levels of CD45 isoforms did not reveal differences between CD45RABC, CD45RB, and CD45RO isoforms. Neither do our CD45RABC/+ or CD45RO/+ mice expressing an altered balance of isoforms show differences following stimulation through CD3/CD28.
The only difference we detect between the two isoforms, expressed in mice with one normally splicing CD45 allele, is that CD45RO/+ cells produce more TNF-α and IFN-γ after PMA-ionomycin stimulation than do CD45RABC/+. Why this difference is seen only after PMA-ionomycin stimulation is not yet clear. However, T-bet has been reported to induce IFN-γ production in Th1 cells stimulated with PMA-ionomycin, but not Ag-APC or IL-12 and IL-18 (31, 39, 40), which is consistent with our results. It is not clear why T-bet is more strongly activated by PMA-ionomycin to induce IFN-γ production, but whatever the mechanism for this marked synergy of T-bet and PMA-ionomycin, this result demonstrates that there is a difference in signaling between CD45RABC/+ and CD45RO/+ cells.
The increased STAT1 phosphorylation and T-bet expression in CD45RO/+ cells further indicates that CD45 isoform expression patterns affect not only cytokine production, but the response to cytokines, because STAT1 phosphorylation is induced by an autocrine pathway involving IFN-γ (31, 39). Our data showing that CD45 regulates cytokine responses are supported by evidence that CD45−/− mice have increased levels of phosphorylation of Jaks and STATs and response to cytokines (27). In this study, for the first time, we show that an excess of the CD45RO isoform preferentially induces TNF-α and IFN-γ production and a bias toward a Th1 response.
Why excess of CD45RO leads to increased TNF-α and IFN-γ production following PMA-ionomycin stimulation is not clear, nor why there is increased STAT1 phosphorylation indicative of an increased response to IFN-γ (31, 39). A possible explanation could be through interactions with other CD45 isoforms. CD45RO has been suggested to homodimerize more efficiently than other isoforms, although recent structural evidence does not support this model (13). However, this does not exclude the possibility of interactions with other CD45 isoforms or molecules distinct from CD45 (35, 37, 38, 41). Most likely, spatiotemporal mechanisms could account for differences in isoform function, and the small size of the CD45RO isoform may allow it to associate more readily with the immunological synapse (14-17).
The most striking effect of altered CD45 isoform expression is on the development of EAE. Both CD45RABC/+ and CD45RO/+ mice showed more rapid onset and increased severity of disease than CD45+/+ mice, with the most severe disease occurring in CD45RO/+ mice. TNF-α and IFN-γ have been shown to influence the severity of disease in this model in a complex fashion, as have other cytokines, such as IL-17, IL-23, and IL-27 (42-46). It is therefore not surprising that CD45RO/+ mice, which have altered regulation of TNF-α and IFN-γ production, show the most severe disease. Interestingly, T cells from A138G variant humans produce more IFN-γ after PMA-ionomycin stimulation (21) as in CD45RO/+ mice, although neither TNF-α production nor the incidence of MS has been studied in A138G individuals. Other mechanisms may also account for the more severe disease in CD45 Tg mice, including a direct effect of CD45 on myelination, as has been reported recently in CD45−/− mice (47). Whatever the mechanisms causing more severe EAE in our Tg mice, the human C77G variant has been associated with MS, while A138G has been shown to be protective in other autoimmune disease, such as Graves' disease and type I diabetes (our unpublished data). It is clear, therefore, that human variants, which like CD45RABC/+ or CD45RO/+ mice show altered phenotype and function, are associated with altered susceptibility to autoimmune diseases.
Reduced CD45 levels and reduced phosphatase activity of CD45 have been reported in systemic lupus erythematosus (48), while MRL mice heterozygous for CD45 express less CD45 on the cell surface and have delayed kinetics of disease development (49). Genetically controlled differences in CD45 isoform distribution in rats are associated with different susceptibility to autoimmune disease (50-52). In cattle (53) and primates (54), CD45 polymorphisms in the extracellular domain have been proposed to be under strong genetic selection, suggesting an important role in pathogen resistance. The human C77G and A138G CD45 variants also demonstrate that subtle changes in CD45 isoform expression have significant effects on immune function and both autoimmune and infectious disease. Taken together, these data indicate that CD45 is an important immunomodulator that influences autoimmunity and infectious disease. The high frequency of A138G in Japan (21, 26), as well as in China, Thailand, Cambodia, Vietnam, and India (our unpublished data), indicates that it may have arisen as a result of selection by pathogens. In any case, A138G is a common allele with a low penetrance, which may have a large impact on disease burden in the human population (55).
In this study, for the first time, we have shown experimentally that altering the combination of CD45 isoforms dramatically affects immune function and disease severity in an autoimmune model. The data also show that the mechanism is an altered threshold for TCR signaling and altered cytokine production and response. This indicates that manipulating the patterns of CD45 expression or signaling pathways that it modulates will be a useful therapeutic strategy. Mice with altered combinations of CD45 isoforms, such as the CD45RABC/+ or CD45RO/+ Tg described in this work, will provide models for future in-depth studies of the underlying mechanisms.
Acknowledgments
We thank Dr. Agnes Le Bon for helpful discussions, and Jenny Piercy for technical assistance.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Abbreviations used in this paper: MS, multiple sclerosis; EAE, experimental autoimmune encephalomyelitis; Tg, transgenic.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Hermiston ML, Xu Z, Weiss A. CD45: a critical regulator of signaling thresholds in immune cells. Annu. Rev. Immunol. 2003;21:107–137. doi: 10.1146/annurev.immunol.21.120601.140946. [DOI] [PubMed] [Google Scholar]
- 2.Penninger JM, Irie-Sasaki J, Sasaki T, Oliveira-Dos-Santos AJ. CD45: new jobs for an old acquaintance. Nat. Immunol. 2001;2:389–396. doi: 10.1038/87687. [DOI] [PubMed] [Google Scholar]
- 3.Akbar AN, Terry L, Timms A, Beverley PC, Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J. Immunol. 1988;140:2171–2178. [PubMed] [Google Scholar]
- 4.Powrie F, Mason D. Subsets of rat CD4+ T cells defined by their differential expression of variants of the CD45 antigen: developmental relationships and in vitro and in vivo functions. Curr. Top. Microbiol. Immunol. 1990;159:79–96. doi: 10.1007/978-3-642-75244-5_5. [DOI] [PubMed] [Google Scholar]
- 5.Merkenschlager M, Terry L, Edwards R, Beverley PC. Limiting dilution analysis of proliferative responses in human lymphocyte populations defined by the monoclonal antibody UCHL1: implications for differential CD45 expression in T cell memory formation. Eur. J. Immunol. 1988;18:1653–1661. doi: 10.1002/eji.1830181102. [DOI] [PubMed] [Google Scholar]
- 6.Lee WT, Yin XM, Vitetta ES. Functional and ontogenetic analysis of CD45Rhi and CD45Rlo CD4+ T cells. J. Immunol. 1990;144:3288–3295. [PubMed] [Google Scholar]
- 7.Perillo NL, Pace KE, Seilhamer JJ, Baum LG. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378:736–739. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- 8.Uemura K, Yokota Y, Kozutsumi Y, Kawasaki T. A unique CD45 glycoform recognized by the serum mannan-binding protein in immature thymocytes. J. Biol. Chem. 1996;271:4581–4584. doi: 10.1074/jbc.271.9.4581. [DOI] [PubMed] [Google Scholar]
- 9.Symons A, Cooper DN, Barclay AN. Characterization of the interaction between galectin-1 and lymphocyte glycoproteins CD45 and Thy-1. Glycobiology. 2000;10:559–563. doi: 10.1093/glycob/10.6.559. [DOI] [PubMed] [Google Scholar]
- 10.Baldwin TA, Ostergaard HL. Developmentally regulated changes in glucosidase II association with, and carbohydrate content of, the protein tyrosine phosphatase CD45. J. Immunol. 2001;167:3829–3835. doi: 10.4049/jimmunol.167.7.3829. [DOI] [PubMed] [Google Scholar]
- 11.Irles C, Symons A, Michel F, Bakker TR, Van Der Merwe PA, Acuto O. CD45 ectodomain controls interaction with GEMs and Lck activity for optimal TCR signaling. Nat. Immunol. 2003;4:189–197. doi: 10.1038/ni877. [DOI] [PubMed] [Google Scholar]
- 12.Xu Z, Weiss A. Negative regulation of CD45 by differential homodimerization of the alternatively spliced isoforms. Nat. Immunol. 2002;3:764–771. doi: 10.1038/ni822. [DOI] [PubMed] [Google Scholar]
- 13.Nam HJ, Poy F, Saito H, Frederick CA. Structural basis for the function and regulation of the receptor protein tyrosine phosphatase CD45. J. Exp. Med. 2005;201:441–452. doi: 10.1084/jem.20041890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang M, Moran M, Round J, Low TA, Patel VP, Tomassian T, Hernandez JD, Miceli MC. CD45 signals outside of lipid rafts to promote ERK activation, synaptic raft clustering, and IL-2 production. J. Immunol. 2005;174:1479–1490. doi: 10.4049/jimmunol.174.3.1479. [DOI] [PubMed] [Google Scholar]
- 15.Edmonds SD, Ostergaard HL. Dynamic association of CD45 with detergent-insoluble microdomains in T lymphocytes. J. Immunol. 2002;169:5036–5042. doi: 10.4049/jimmunol.169.9.5036. [DOI] [PubMed] [Google Scholar]
- 16.Freiberg BA, Kupfer H, Maslanik W, Delli J, Kappler J, Zaller DM, Kupfer A. Staging and resetting T cell activation in SMACs. Nat. Immunol. 2002;3:911–917. doi: 10.1038/ni836. [DOI] [PubMed] [Google Scholar]
- 17.Mustelin T, Vang T, Bottini N. Protein tyrosine phosphatases and the immune response. Nat. Rev. Immunol. 2005;5:43–57. doi: 10.1038/nri1530. [DOI] [PubMed] [Google Scholar]
- 18.Tchilian EZ, Wallace DL, Wells RS, Flower DR, Morgan G, Beverley PC. A deletion in the gene encoding the CD45 antigen in a patient with SCID. J. Immunol. 2001;166:1308–1313. doi: 10.4049/jimmunol.166.2.1308. [DOI] [PubMed] [Google Scholar]
- 19.Byth KF, Conroy LA, Howlett S, Smith AJ, May J, Alexander DR, Holmes N. CD45-null transgenic mice reveal a positive regulatory role for CD45 in early thymocyte development, in the selection of CD4+CD8+ thymocytes, and B cell maturation. J. Exp. Med. 1996;183:1707–1718. doi: 10.1084/jem.183.4.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobsen M, Schweer D, Ziegler A, Gaber R, Schock S, Schwinzer R, Wonigeit K, Lindert RB, Kantarci O, Schaefer-Klein J, et al. A point mutation in PTPRC is associated with the development of multiple sclerosis. Nat. Genet. 2000;26:495–499. doi: 10.1038/82659. [DOI] [PubMed] [Google Scholar]
- 21.Boxall S, Stanton T, Hirai K, Ward V, Yasui T, Tahara H, Tamori A, Nishiguchi S, Shiomi S, Ishiko O, et al. Disease associations and altered immune function in CD45 138G variant carriers. Hum. Mol. Genet. 2004;13:2377–2384. doi: 10.1093/hmg/ddh276. [DOI] [PubMed] [Google Scholar]
- 22.Vogel A, Strassburg CP, Manns MP. 77 C/G mutation in the tyrosine phosphatase CD45 gene and autoimmune hepatitis: evidence for a genetic link. Genes Immun. 2003;4:79–81. doi: 10.1038/sj.gene.6363918. [DOI] [PubMed] [Google Scholar]
- 23.Tchilian EZ, Wallace DL, Dawes R, Imami N, Burton C, Gotch F, Beverley PC. A point mutation in CD45 may be associated with HIV-1 infection. AIDS. 2001;15:1892–1894. doi: 10.1097/00002030-200109280-00024. [DOI] [PubMed] [Google Scholar]
- 24.Schwinzer R, Witte T, Hundrieser J, Ehlers S, Momot T, Hunzelmann N, Krieg T, Schmidt RE, Wonigeit K. Enhanced frequency of a PTPRC (CD45) exon A mutation (77C3→G) in systemic sclerosis. Genes Immun. 2003;4:168–169. doi: 10.1038/sj.gene.6363894. [DOI] [PubMed] [Google Scholar]
- 25.Vyshkina T, Leist TP, Shugart YY, Kalman B. CD45 (PTPRC) as a candidate gene in multiple sclerosis. Mult. Scler. 2004;10:614–617. doi: 10.1191/1352458504ms1115oa. [DOI] [PubMed] [Google Scholar]
- 26.Stanton T, Boxall S, Hirai K, Dawes R, Tonks S, Yasui T, Kanaoka Y, Yuldasheva N, Ishiko O, Bodmer W, et al. A high-frequency polymorphism in exon 6 of the CD45 tyrosine phosphatase gene (PTPRC) resulting in altered isoform expression. Proc. Natl. Acad. Sci. USA. 2003;100:5997–6002. doi: 10.1073/pnas.0931490100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irie-Sasaki J, Sasaki T, Matsumoto W, Opavsky A, Cheng M, Welstead G, Griffiths E, Krawczyk C, Richardson CD, Aitken K, et al. CD45 is a JAK phosphatase and negatively regulates cytokine receptor signalling. Nature. 2001;409:349–354. doi: 10.1038/35053086. [DOI] [PubMed] [Google Scholar]
- 28.Huntington ND, Xu Y, Nutt SL, Tarlinton DM. A requirement for CD45 distinguishes Ly49D-mediated cytokine and chemokine production from killing in primary natural killer cells. J. Exp. Med. 2005;201:1421–1433. doi: 10.1084/jem.20042294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tchilian EZ, Dawes R, Hyland L, Montoya M, Le Bon A, Borrow P, Hou S, Tough D, Beverley PC. Altered CD45 isoform expression affects lymphocyte function in CD45 Tg mice. Int. Immunol. 2004;16:1323–1332. doi: 10.1093/intimm/dxh135. [DOI] [PubMed] [Google Scholar]
- 30.Mendel I, Kerlero de Rosbo N, Ben-Nun A. A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2b mice: fine specificity and T cell receptor Vβ expression of encephalitogenic T cells. Eur. J. Immunol. 1995;25:1951–1959. doi: 10.1002/eji.1830250723. [DOI] [PubMed] [Google Scholar]
- 31.Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY, Murphy TL, Murphy KM. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat. Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 32.Ballerini C, Rosati E, Salvetti M, Ristori G, Cannoni S, Biagioli T, Massacesi L, Sorbi S, Vergelli M. Protein tyrosine phosphatase receptor-type C exon 4 gene mutation distribution in an Italian multiple sclerosis population. Neurosci. Lett. 2002;328:325–327. doi: 10.1016/s0304-3940(02)00565-7. [DOI] [PubMed] [Google Scholar]
- 33.Ogilvy S, Louis-Dit-Sully C, Cooper J, Cassady RL, Alexander DR, Holmes N. Either of the CD45RB and CD45RO isoforms are effective in restoring T cell, but not B cell, development and function in CD45-null mice. J. Immunol. 2003;171:1792–1800. doi: 10.4049/jimmunol.171.4.1792. [DOI] [PubMed] [Google Scholar]
- 34.Medzhitov R, Janeway CA., Jr. Innate immunity: impact on the adaptive immune response. Curr. Opin. Immunol. 1997;9:4–9. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- 35.Novak TJ, Farber D, Leitenberg D, Hong SC, Johnson P, Bottomly K. Isoforms of the transmembrane tyrosine phosphatase CD45 differentially affect T cell recognition. Immunity. 1994;1:109–119. doi: 10.1016/1074-7613(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 36.Onodera H, Motto DG, Koretzky GA, Rothstein DM. Differential regulation of activation-induced tyrosine phosphorylation and recruitment of SLP-76 to Vav by distinct isoforms of the CD45 protein-tyrosine phosphatase. J. Biol. Chem. 1996;271:22225–22230. doi: 10.1074/jbc.271.36.22225. [DOI] [PubMed] [Google Scholar]
- 37.Dornan S, Sebestyen Z, Gamble J, Nagy P, Bodnar A, Alldridge L, Doe S, Holmes N, Goff LK, Beverley P, et al. Differential association of CD45 isoforms with CD4 and CD8 regulates the actions of specific pools of p56lck tyrosine kinase in T cell antigen receptor signal transduction. J. Biol. Chem. 2002;277:1912–1918. doi: 10.1074/jbc.M108386200. [DOI] [PubMed] [Google Scholar]
- 38.Leitenberg D, Novak TJ, Farber D, Smith BR, Bottomly K. The extracellular domain of CD45 controls association with the CD4-T cell receptor complex and the response to antigen-specific stimulation. J. Exp. Med. 1996;183:249–259. doi: 10.1084/jem.183.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM, Kung AL, Cereb N, Yao TP, Yang SY, Reiner SL. Role of T-bet in commitment of Th1 cells before IL-12-dependent selection. Science. 2001;292:1907–1910. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 40.Robinson D, Shibuya K, Mui A, Zonin F, Murphy E, Sana T, Hartley SB, Menon S, Kastelein R, Bazan F, O'Garra A. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-γ production and activates IRAK and NFκB. Immunity. 1997;7:571–581. doi: 10.1016/s1074-7613(00)80378-7. [DOI] [PubMed] [Google Scholar]
- 41.Geng X, Tang RH, Law SK, Tan SM. Integrin CD11a cytoplasmic tail interacts with the CD45 membrane-proximal protein tyrosine phosphatase domain 1. Immunology. 2005;115:347–357. doi: 10.1111/j.1365-2567.2005.02175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Panitch HS, Hirsch RL, Haley AS, Johnson KP. Exacerbations of multiple sclerosis in patients treated with γ interferon. Lancet. 1987;1:893–895. doi: 10.1016/s0140-6736(87)92863-7. [DOI] [PubMed] [Google Scholar]
- 43.Willenborg DO, Fordham SA, Staykova MA, Ramshaw IA, Cowden WB. IFN-γ is critical to the control of murine autoimmune encephalomyelitis and regulates both in the periphery and in the target tissue: a possible role for nitric oxide. J. Immunol. 1999;163:5278–5286. [PubMed] [Google Scholar]
- 44.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat. Rev. Immunol. 2005;5:521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 45.Selmaj K, Raine CS, Cross AH. Anti-tumor necrosis factor therapy abrogates autoimmune demyelination. Ann. Neurol. 1991;30:694–700. doi: 10.1002/ana.410300510. [DOI] [PubMed] [Google Scholar]
- 46.Ruddle NH, Bergman CM, McGrath KM, Lingenheld EG, Grunnet ML, Padula SJ, Clark RB. An antibody to lymphotoxin and tumor necrosis factor prevents transfer of experimental allergic encephalomyelitis. J. Exp. Med. 1990;172:1193–1200. doi: 10.1084/jem.172.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakahara J, Seiwa C, Tan-Takeuchi K, Gotoh M, Kishihara K, Ogawa M, Asou H, Aiso S. Involvement of CD45 in central nervous system myelination. Neurosci. Lett. 2005;379:116–121. doi: 10.1016/j.neulet.2004.12.066. [DOI] [PubMed] [Google Scholar]
- 48.Takeuchi T, Pang M, Amano K, Koide J, Abe T. Reduced protein tyrosine phosphatase (PTPase) activity of CD45 on peripheral blood lymphocytes in patients with systemic lupus erythematosus (SLE) Clin. Exp. Immunol. 1997;109:20–26. doi: 10.1046/j.1365-2249.1997.4371334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brooks WP, Lynes MA. Effects of hemizygous CD45 expression in the autoimmune FasLgld/gld syndrome. Cell. Immunol. 2001;212:24–34. doi: 10.1006/cimm.2001.1845. [DOI] [PubMed] [Google Scholar]
- 50.Subra JF, Cautain B, Xystrakis E, Mas M, Lagrange D, van der Heijden H, van de Gaar MJ, Druet P, Fournie GJ, Saoudi A, Damoiseaux J. The balance between CD45RChigh and CD45RClow CD4 T cells in rats is intrinsic to bone marrow-derived cells and is genetically controlled. J. Immunol. 2001;166:2944–2952. doi: 10.4049/jimmunol.166.5.2944. [DOI] [PubMed] [Google Scholar]
- 51.Xystrakis E, Cavailles P, Dejean AS, Cautain B, Colacios C, Lagrange D, van de Gaar MJ, Bernard I, Gonzalez-Dunia D, Damoiseaux J, et al. Functional and genetic analysis of two CD8 T cell subsets defined by the level of CD45RC expression in the rat. J. Immunol. 2004;173:3140–3147. doi: 10.4049/jimmunol.173.5.3140. [DOI] [PubMed] [Google Scholar]
- 52.Seddon B, Mason D. Regulatory T cells in the control of autoimmunity: the essential role of transforming growth factor β and interleukin 4 in the prevention of autoimmune thyroiditis in rats by peripheral CD4+CD45RC− cells and CD4+CD8− thymocytes. J. Exp. Med. 1999;189:279–288. doi: 10.1084/jem.189.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ballingall KT, Waibochi L, Holmes EC, Woelk CH, MacHugh ND, Lutje V, McKeever DJ. The CD45 locus in cattle: allelic polymorphism and evidence for exceptional positive natural selection. Immunogenetics. 2001;52:276–283. doi: 10.1007/s002510000276. [DOI] [PubMed] [Google Scholar]
- 54.Filip LC, Mundy NI. Rapid evolution by positive Darwinian selection in the extracellular domain of the abundant lymphocyte protein CD45 in primates. Mol. Biol. Evol. 2004;21:1504–1511. doi: 10.1093/molbev/msh111. [DOI] [PubMed] [Google Scholar]
- 55.Rioux JD, Abbas AK. Paths to understanding the genetic basis of autoimmune disease. Nature. 2005;435:584–589. doi: 10.1038/nature03723. [DOI] [PubMed] [Google Scholar]