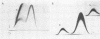Abstract
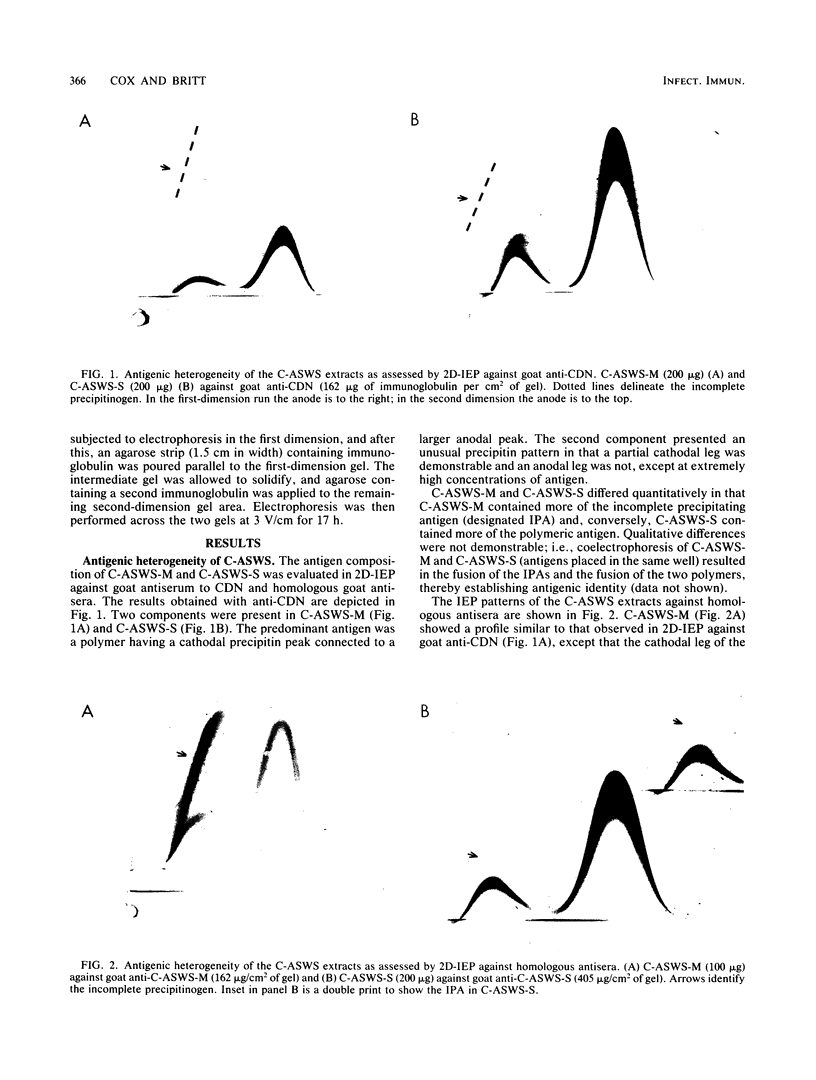
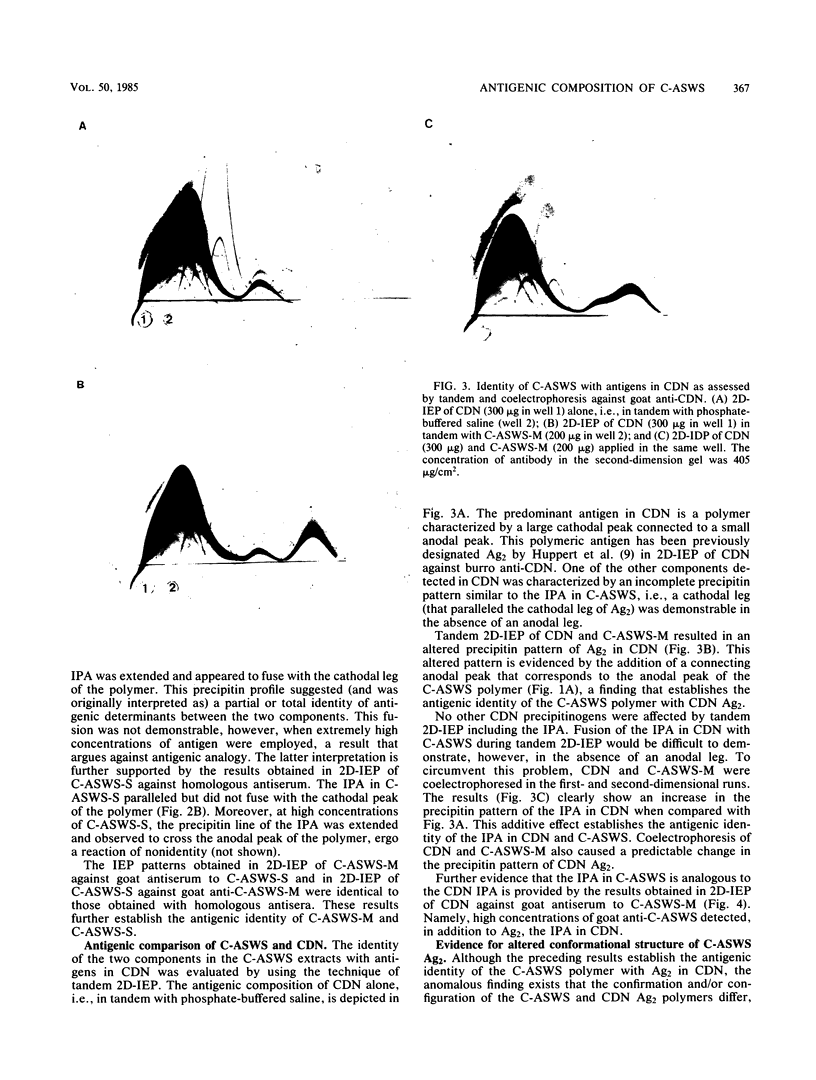
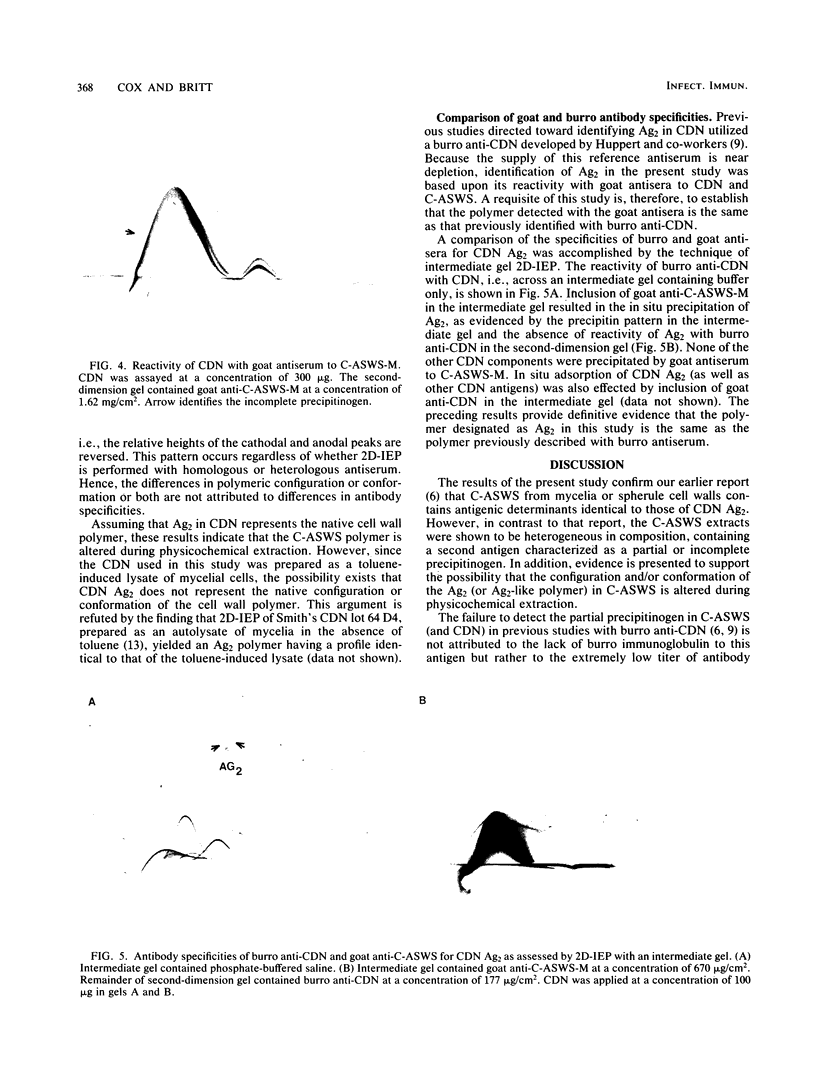
The antigenic composition of an alkali-soluble, water-soluble cell wall extract of Coccidioides immitis, designated C-ASWS, was assessed by two-dimensional immunoelectrophoresis against goat antisera to C-ASWS and coccidioidin. The results established that C-ASWS from mycelia or spherule cell walls is heterogeneous in composition, containing two distinct antigenic components. One is present as a polymer that is antigenically identical to a polymeric antigen in coccidioidin, designated antigen 2. The other component detected in C-ASWS presented an unusual precipitin pattern in that a cathodal leg was demonstrable in the absence of an anodal leg. This incomplete precipitinogen was also detected in coccidioidin. In addition to the finding that C-ASWS is antigenically heterogeneous, the results provide evidence that the conformational and/or configurational structure of the C-ASWS antigen 2 (or antigen 2-like polymer) is altered during physicochemical extraction. This conclusion is based upon the finding that the immunoelectrophoretic profile of the C-ASWS polymer differs from that of coccidioidin antigen 2. The C-ASWS polymer is characterized by having a small cathodal precipitin peak connected to a large anodal peak, whereas coccidioidin antigen 2 is characterized by a predominant cathodal peak.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelsen N. H. Intermediate gel in crossed and in fused rocket immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:71–77. doi: 10.1111/j.1365-3083.1973.tb03782.x. [DOI] [PubMed] [Google Scholar]
- Ballou C. Structure and biosynthesis of the mannan component of the yeast cell envelope. Adv Microb Physiol. 1976;14(11):93–158. doi: 10.1016/s0065-2911(08)60227-1. [DOI] [PubMed] [Google Scholar]
- Cox R. A., Arnold D. R. Immunoglobulin E in coccidioidomycosis. J Immunol. 1979 Jul;123(1):194–200. [PubMed] [Google Scholar]
- Cox R. A., Brummer E., Lecara G. In vitro lymphocyte responses of coccidioidin skin test-positive and -negative persons to coccidioidin, spherulin, and a coccidioides cell wall antigen. Infect Immun. 1977 Mar;15(3):751–755. doi: 10.1128/iai.15.3.751-755.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A., Huppert M., Starr P., Britt L. A. Reactivity of alkali-soluble, water-soluble cell wall antigen of Coccidioides immitis with anti-Coccidioides immunoglobulin M precipitin antibody. Infect Immun. 1984 Feb;43(2):502–507. doi: 10.1128/iai.43.2.502-507.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A., Mead C. G., Pavey E. F. Comparisons of mycelia- and spherule-derived antigens in cellular immune assays of Coccidioides immitis-infected guinea pigs. Infect Immun. 1981 Feb;31(2):687–692. doi: 10.1128/iai.31.2.687-692.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A., Vivas J. R. Spectrum of in vivo and in vitro cell-mediated immune responses in coccidioidomycosis. Cell Immunol. 1977 Jun 1;31(1):130–141. doi: 10.1016/0008-8749(77)90012-0. [DOI] [PubMed] [Google Scholar]
- Huppert M., Spratt N. S., Vukovich K. R., Sun S. H., Rice E. H. Antigenic analysis of coccidioidin and spherulin determined by two-dimensional immunoelectrophoresis. Infect Immun. 1978 May;20(2):541–551. doi: 10.1128/iai.20.2.541-551.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll J. Tandem-crossed immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:57–59. [PubMed] [Google Scholar]
- Lecara G., Cox R. A., Simpson R. B. Coccidioides immitis vaccine: potential of an alkali-soluble, water-soluble cell wall antigen. Infect Immun. 1983 Jan;39(1):473–475. doi: 10.1128/iai.39.1.473-475.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAPPAGIANIS D., SMITH C. E., KOBAYASHI G. S., SAITO M. T. Studies of antigens from young mycelia of Coccidioides immitis. J Infect Dis. 1961 Jan-Feb;108:35–44. doi: 10.1093/infdis/108.1.35. [DOI] [PubMed] [Google Scholar]
- Ward E. R., Jr, Cox R. A., Schmitt J. A., Jr, Huppert M., Sun S. H. Delayed-type hypersensitivity responses to a cell wall fraction of the mycelial phase of Coccidioides immitis. Infect Immun. 1975 Nov;12(5):1093–1097. doi: 10.1128/iai.12.5.1093-1097.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeke B. Crossed immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:47–56. doi: 10.1111/j.1365-3083.1973.tb03778.x. [DOI] [PubMed] [Google Scholar]
- Wheat R., Scheer E. Cell walls of Coccidioides immitis: neutral sugars of aqueous alkaline extract polymers. Infect Immun. 1977 Jan;15(1):340–341. doi: 10.1128/iai.15.1.340-341.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]