Abstract
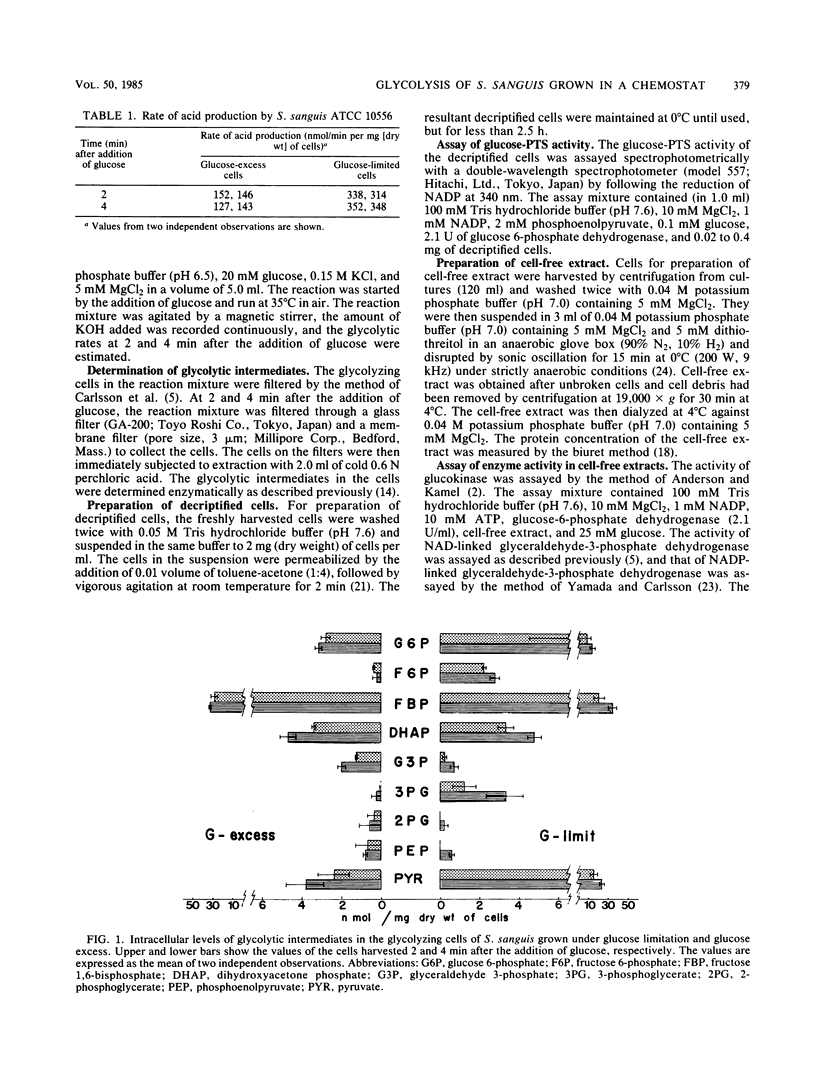
The biochemical mechanisms of the acidogenic potential of Streptococcus sanguis ATCC 10556 grown in glucose-excess and glucose-limited continuous culture were studied. The rate of acid production during the glucose metabolism by the cells grown under glucose limitation (glucose-limited cells) was 2.1 to 2.6 times that by the cells grown in an excess of glucose (glucose-excess cells). When the glucose-limited cells were metabolizing glucose, intracellular concentrations of glucose 6-phosphate, fructose 6-phosphate, 3-phosphoglycerate, and pyruvate were higher, and that of glyceraldehyde 3-phosphate was lower, than those when the glucose-excess cells were metabolizing glucose. The levels of fructose 1,6-bisphosphate and dihydroxyacetone phosphate were not significantly different between these cells. The activities of glucose-phosphoenolpyruvate phosphotransferase system in decriptified cells and glyceraldehyde-3-phosphate dehydrogenase in cell-free extracts of the glucose-limited cells were higher than those in the glucose-excess cells. The activities of glucokinase, phosphoglycerate kinase, and pyruvate kinase in cell-free extracts of these cells were not different significantly. We conclude that the high glycolytic activity of the glucose-limited cells results from the increase in the synthesis of glucose-phosphoenolpyruvate phosphotransferase and glyceraldehyde-3-phosphate dehydrogenase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbe K., Takahashi S., Yamada T. Purification and properties of pyruvate kinase from Streptococcus sanguis and activator specificity of pyruvate kinase from oral streptococci. Infect Immun. 1983 Mar;39(3):1007–1014. doi: 10.1128/iai.39.3.1007-1014.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. T., Wittenberger C. L. The occurrence of multiple glyceraldehyde-3-phosphate dehydrogenases in cariogenic streptococci. Biochem Biophys Res Commun. 1971 Apr 2;43(1):217–224. doi: 10.1016/s0006-291x(71)80110-9. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Griffith C. J. Fermentation products and bacterial yields in glucose-limited and nitrogen-limited cultures of streptococci. Arch Oral Biol. 1974 Dec;19(12):1105–1109. doi: 10.1016/0003-9969(74)90238-6. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Iwami Y., Yamada T. Hydrogen peroxide excretion by oral streptococci and effect of lactoperoxidase-thiocyanate-hydrogen peroxide. Infect Immun. 1983 Apr;40(1):70–80. doi: 10.1128/iai.40.1.70-80.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J. A. A biochemical approach to the control of dental caries. Biochem Soc Trans. 1977;5(4):1232–1239. doi: 10.1042/bst0051232. [DOI] [PubMed] [Google Scholar]
- Edwardsson S. Characteristics of caries-inducing human streptococci resembling Streptococcus mutans. Arch Oral Biol. 1968 Jun;13(6):637–646. doi: 10.1016/0003-9969(68)90142-8. [DOI] [PubMed] [Google Scholar]
- Eisenberg A. D., Bender G. R., Marquis R. E. Reduction in the aciduric properties of the oral bacterium Streptococcus mutans GS-5 by fluoride. Arch Oral Biol. 1980;25(2):133–135. doi: 10.1016/0003-9969(80)90089-8. [DOI] [PubMed] [Google Scholar]
- Ellwood D. C., Phipps P. J., Hamilton I. R. Effect of growth rate and glucose concentration on the activity of the phosphoenolpyruvate phosphotransferase system in Streptococcus mutans Ingbritt grown in continuous culture. Infect Immun. 1979 Feb;23(2):224–231. doi: 10.1128/iai.23.2.224-231.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., Ellwood D. C. Carbohydrate metabolism by Actinomyces viscosus growing in continuous culture. Infect Immun. 1983 Oct;42(1):19–26. doi: 10.1128/iai.42.1.19-26.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., Ellwood D. C. Effects of fluoride on carbohydrate metabolism by washed cells of Streptococcus mutans grown at various pH values in a chemostat. Infect Immun. 1978 Feb;19(2):434–442. doi: 10.1128/iai.19.2.434-442.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., Phipps P. J., Ellwood D. C. Effect of growth rate and glucose concentration on the biochemical properties of Streptococcus mutans Ingbritt in continuous culture. Infect Immun. 1979 Dec;26(3):861–869. doi: 10.1128/iai.26.3.861-869.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwami Y., Yamada T., Araya S. Glycolytic intermediates in Streptococcus mutans PK 1. Arch Oral Biol. 1975 Oct;20(10):695–697. doi: 10.1016/0003-9969(75)90140-5. [DOI] [PubMed] [Google Scholar]
- Iwami Y., Yamada T. Rate-limiting steps of the glycolytic pathway in the oral bacteria Streptococcus mutans and Streptococcus sanguis and the influence of acidic pH on the glucose metabolism. Arch Oral Biol. 1980;25(3):163–169. doi: 10.1016/0003-9969(80)90015-1. [DOI] [PubMed] [Google Scholar]
- Kanapka J. A., Hamilton I. R. Fluoride inhibition of enolase activity in vivo and its relationship to the inhibition of glucose-6-P formation in Streptococcus salivarius. Arch Biochem Biophys. 1971 Sep;146(1):167–174. doi: 10.1016/s0003-9861(71)80053-x. [DOI] [PubMed] [Google Scholar]
- Krasse B., Jordan H. V., Edwardsson S., Svensson I., Trell L. The occurrence of certain "caries-inducing" streptococci in human dental plaque material with special reference to frequency and activity of caries. Arch Oral Biol. 1968 Aug;13(8):911–918. doi: 10.1016/0003-9969(68)90006-x. [DOI] [PubMed] [Google Scholar]
- Krietsch W. K., Bücher T. 3-phosphoglycerate kinase from rabbit sceletal muscle and yeast. Eur J Biochem. 1970 Dec;17(3):568–580. doi: 10.1111/j.1432-1033.1970.tb01202.x. [DOI] [PubMed] [Google Scholar]
- Marsh P. D., Williamson M. I., Keevil C. W., McDermid A. S., Ellwood D. C. Influence of sodium and potassium ions on acid production by washed cells of Streptococcus mutans ingbritt and Streptococcus sanguis NCTC 7865 grown in a chemostat. Infect Immun. 1982 May;36(2):476–483. doi: 10.1128/iai.36.2.476-483.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickelson M. N. Effect of lactoperoxidase and thiocyanate on the growth of Streptococcus pyogenes and Streptococcus agalactiae in a chemically defined culture medium. J Gen Microbiol. 1966 Apr;43(1):31–43. doi: 10.1099/00221287-43-1-31. [DOI] [PubMed] [Google Scholar]
- Slee A. M., Tanzer J. M. Phosphoenolpyruvate-dependent sucrose phosphotransferase activity in Streptococcus mutans NCTC 10449. Infect Immun. 1979 Jun;24(3):821–828. doi: 10.1128/iai.24.3.821-828.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Krichevsky M. I., Keyes P. H. The metabolic fate of glucose catabolized by a washed stationary phase caries-conducive streptococcus. Caries Res. 1969;3(2):167–177. doi: 10.1159/000259580. [DOI] [PubMed] [Google Scholar]
- Yamada T., Carlsson J. Phosphoenolpyruvate carboxylase and ammonium metabolism in oral streptococci. Arch Oral Biol. 1973 Jul;18(7):799–812. doi: 10.1016/0003-9969(73)90051-4. [DOI] [PubMed] [Google Scholar]
- Yamada T., Carlsson J. Regulation of lactate dehydrogenase and change of fermentation products in streptococci. J Bacteriol. 1975 Oct;124(1):55–61. doi: 10.1128/jb.124.1.55-61.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


