Abstract
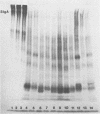
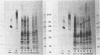
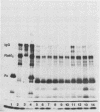
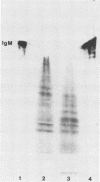
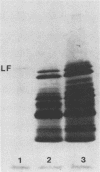
The structural integrity of immunoglobulin A (IgA), IgG, and IgM and lactoferrin in dental plaque fluid samples from two populations of Colombian children with contrasting levels of dental caries was examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by electrophoretic transfer to nitrocellulose. The immune factors or their fragments or both were detected with monospecific antibody conjugated with horseradish peroxidase. All the immune factors examined were extensively degraded, although there appeared to be small amounts of intact IgA and IgG in some samples. Analysis of the samples with antibody to secretory component showed that secretory IgA as well as serum IgA was degraded. IgG appeared to be cleaved into two major fragments, one fragment having a relative mobility similar to the F(ab')2 fragment of IgG and the other a relative mobility slightly greater than Fc. IgM and lactoferrin were virtually completely degraded. There was no apparent relationship between the fragmentation patterns of IgA and IgG in the plaque fluid samples from the two communities and their susceptibility to dental caries.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baici A., Knöpfel M., Fehr K. Cathepsin G from human polymorphonuclear leukocytes cleaves human IgM. Mol Immunol. 1982 May;19(5):719–727. doi: 10.1016/0161-5890(82)90373-x. [DOI] [PubMed] [Google Scholar]
- Bowen W. H., Velez H., Aguila M., Velasquez H., Sierra L. I., Gillespie G. The microbiology and biochemistry of plaque, saliva, and drinking water from two communities with contrasting levels of caries in Colombia, S.A. J Dent Res. 1977 Oct;56(Spec No):C32–C39. doi: 10.1177/002203457705600314011. [DOI] [PubMed] [Google Scholar]
- Brown W. R., Newcomb R. W., Ishizaka K. Proteolytic degradation of exocrine and serum immunoglobulins. J Clin Invest. 1970 Jul;49(7):1374–1380. doi: 10.1172/JCI106354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M. F., Hsu S. D., Baum B. J., Bowen W. H., Sierra L. I., Aquirre M., Gillespie G. Specific and nonspecific immune factors in dental plaque fluid and saliva from young and old populations. Infect Immun. 1981 Mar;31(3):998–1002. doi: 10.1128/iai.31.3.998-1002.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPaola C., Herrera M. S., Mandel I. D. Host proteins in dental plaques of caries-resistant versus caries-susceptible human groups. Arch Oral Biol. 1984;29(5):353–355. doi: 10.1016/0003-9969(84)90159-6. [DOI] [PubMed] [Google Scholar]
- Gheţie V., Mota G. The decrease of human colostral immunoglobulin A resistance to papain action after gradual release of the secretory component. Immunochemistry. 1973 Dec;10(12):839–840. doi: 10.1016/0019-2791(73)90188-2. [DOI] [PubMed] [Google Scholar]
- Kilian M. Degradation of immunoglobulins A2, A2, and G by suspected principal periodontal pathogens. Infect Immun. 1981 Dec;34(3):757–765. doi: 10.1128/iai.34.3.757-765.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindh E. Increased risistance of immunoglobulin A dimers to proteolytic degradation after binding of secretory component. J Immunol. 1975 Jan;114(1 Pt 2):284–286. [PubMed] [Google Scholar]
- Margolis J., Kenrick K. C. Polyacrylamide gel-electrophoresis across a molecular sieve gradient. Nature. 1967 Jun 24;214(5095):1334–1336. doi: 10.1038/2141334a0. [DOI] [PubMed] [Google Scholar]
- Margolis J., Kenrick K. G. Polyacrylamide gel electrophoresis in a continuous molecular sieve gradient. Anal Biochem. 1968 Oct 24;25(1):347–362. doi: 10.1016/0003-2697(68)90109-7. [DOI] [PubMed] [Google Scholar]
- Plaut A. G., Gilbert J. V., Wistar R., Jr Loss of antibody activity in human immunoglobulin A exposed extracellular immunoglobulin A proteases of Neisseria gonorrhoeae and Streptococcus sanguis. Infect Immun. 1977 Jul;17(1):130–135. doi: 10.1128/iai.17.1.130-135.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaut A. G. The IgA1 proteases of pathogenic bacteria. Annu Rev Microbiol. 1983;37:603–622. doi: 10.1146/annurev.mi.37.100183.003131. [DOI] [PubMed] [Google Scholar]
- Söder P. O. Proteolytic activity in the oral cavity: proteolytic enzymes from human saliva and dental plaque material. J Dent Res. 1972 Mar-Apr;51(2):389–393. doi: 10.1177/00220345720510022601. [DOI] [PubMed] [Google Scholar]
- Tatevossian A., Newbrun E. Electrophoretic and immunoelectrophoretic studies of proteins in the aqueous phase of human dental plaque. Arch Oral Biol. 1981;26(4):275–280. doi: 10.1016/0003-9969(81)90047-9. [DOI] [PubMed] [Google Scholar]
- Taubman M. A. Immunoglobulins of human dental plaque. Arch Oral Biol. 1974 Jun;19(6):439–446. doi: 10.1016/0003-9969(74)90149-6. [DOI] [PubMed] [Google Scholar]
- Taubman M. A., Smith D. J., Murray R. Immunoglobulin susceptibility to proteolytic effects of human dental plaque extracts. Arch Oral Biol. 1978;23(11):949–955. doi: 10.1016/0003-9969(78)90248-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]