Abstract
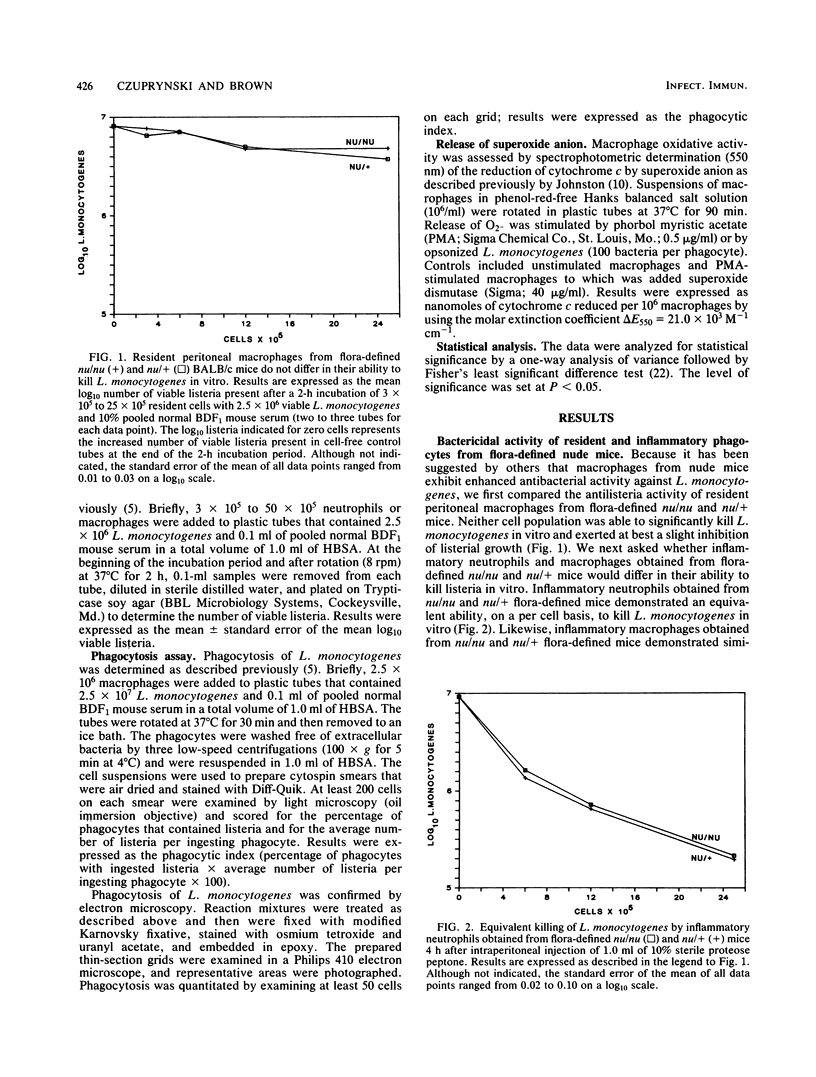
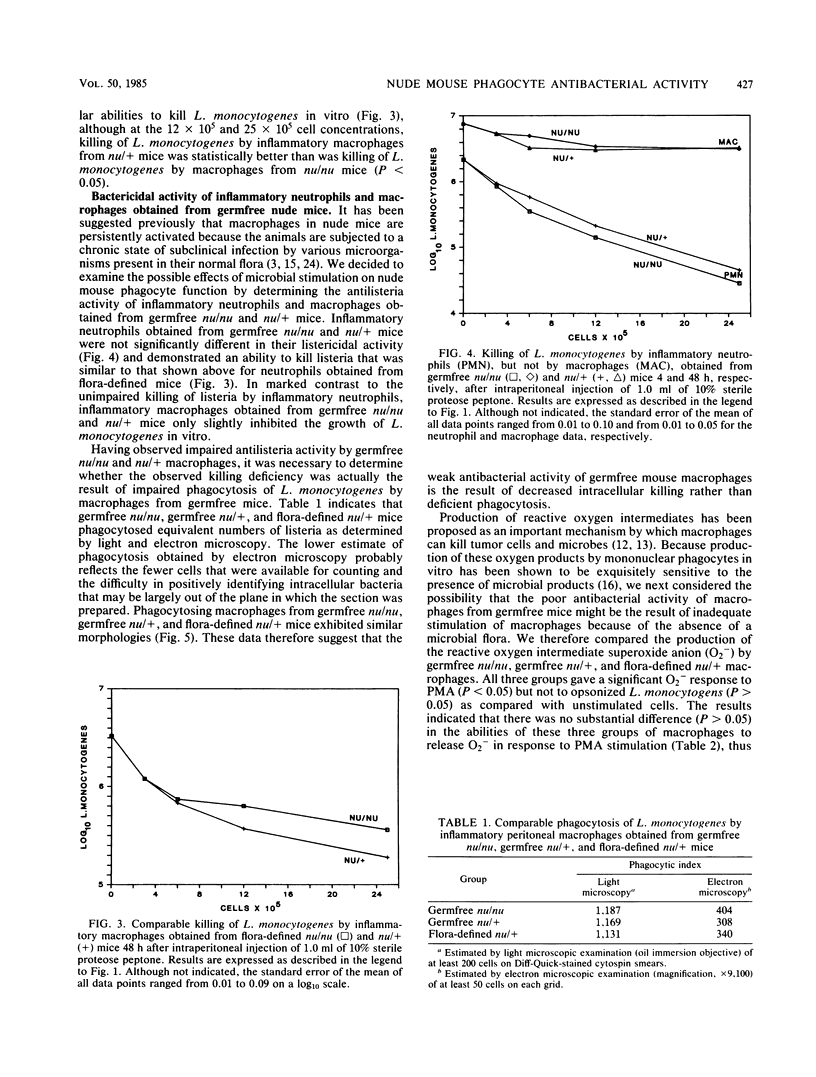
In this study we directly compared the in vitro antibacterial activities of resident and inflammatory phagocytes obtained from athymic (nu/nu) and euthymic (nu/+) mice. Resident peritoneal macrophages obtained from flora-defined nu/nu and nu/+ mice both demonstrated little ability to restrict the growth of Listeria monocytogenes in vitro. Inflammatory peritoneal neutrophils and macrophages obtained from flora-defined nu/nu and nu/+ mice did not differ in their ability to kill L. monocytogenes in vitro. Likewise, inflammatory peritoneal neutrophils obtained from germfree nu/nu and nu/+ mice killed equivalent numbers of listeria. In marked contrast, however, inflammatory macrophages obtained from germfree nu/nu and nu/+ mice demonstrated very limited antilisteria activity in vitro. The reduced antilisteria activity of macrophages from germfree nu/nu and nu/+ mice was associated neither with reduced phagocytosis of L. monocytogenes nor with an inability to generate an oxidative response. Unlike those of previous reports, these data suggest that resident and inflammatory phagocytes obtained from athymic mice that were maintained under conditions such that they were not subjected to underlying infections are not constitutively activated for enhanced antibacterial activity. Furthermore, these data suggest that stimulation of macrophages by products of the bacterial flora is required for the expression of macrophage antibacterial activity.
Full text
PDF
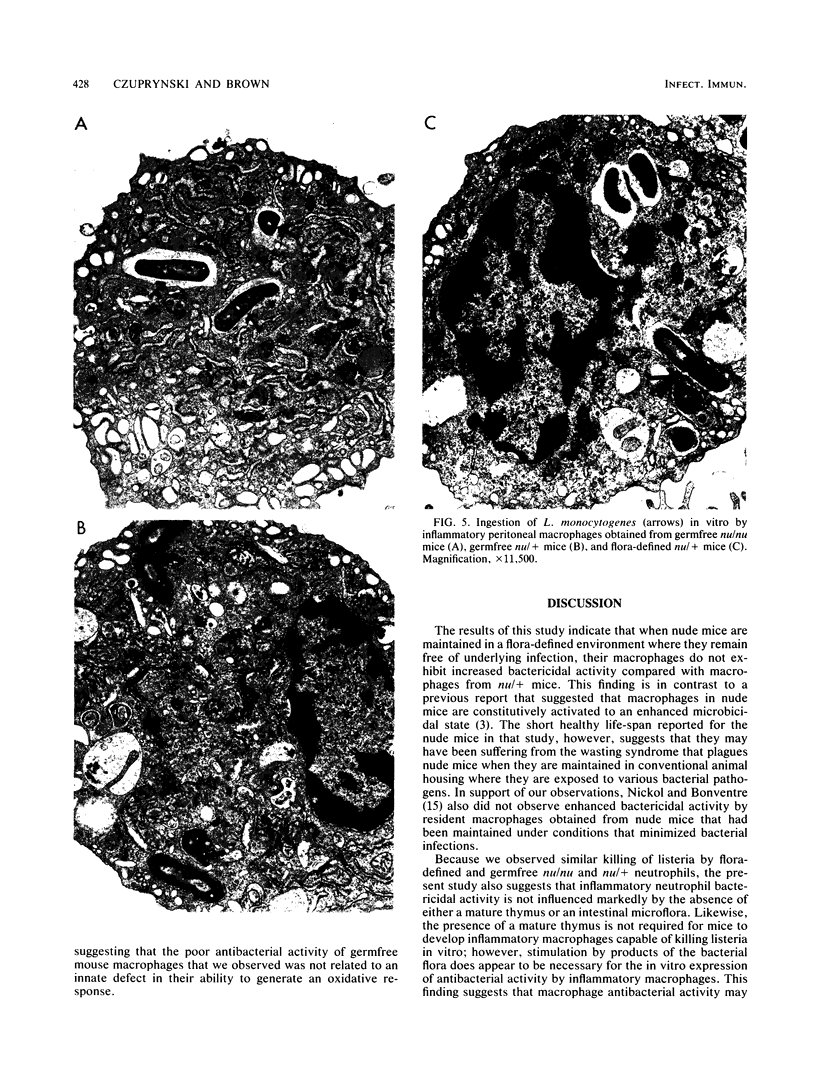
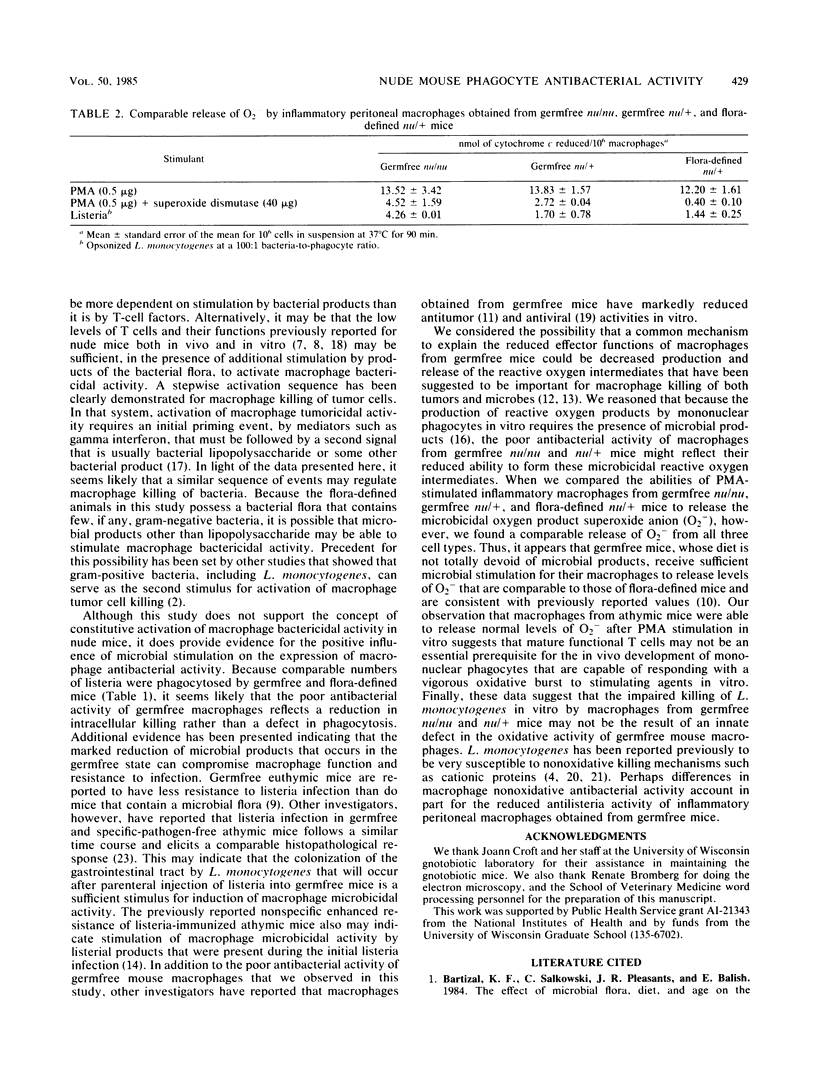

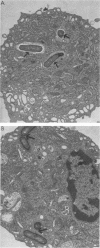
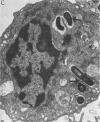
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Celada A., Gray P. W., Rinderknecht E., Schreiber R. D. Evidence for a gamma-interferon receptor that regulates macrophage tumoricidal activity. J Exp Med. 1984 Jul 1;160(1):55–74. doi: 10.1084/jem.160.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C., Waller R. Activated macrophages in congenitally athymic "nude mice" and in lethally irradiate mice. J Immunol. 1975 Sep;115(3):844–847. [PubMed] [Google Scholar]
- Czuprynski C. J., Balish E. Killing of Listeria monocytogens by conventional and germfree rat sera. Infect Immun. 1981 Aug;33(2):348–354. doi: 10.1128/iai.33.2.348-354.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuprynski C. J., Henson P. M., Campbell P. A. Killing of Listeria monocytogenes by inflammatory neutrophils and mononuclear phagocytes from immune and nonimmune mice. J Leukoc Biol. 1984 Feb;35(2):193–208. doi: 10.1002/jlb.35.2.193. [DOI] [PubMed] [Google Scholar]
- Emmerling P., Finger H., Bockemühl J. Listeria monocytogenes infection in nude mice. Infect Immun. 1975 Aug;12(2):437–439. doi: 10.1128/iai.12.2.437-439.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Union N. A., Baker P. E., Smith K. A. The in vitro generation and sustained culture of nude mouse cytolytic T-lymphocytes. J Exp Med. 1979 Jun 1;149(6):1460–1476. doi: 10.1084/jem.149.6.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Saito K. Congenitally athymic nude (nu/nu) mice have Thy-1-bearing immunocompetent helper T cells in their peritoneal cavity. J Exp Med. 1980 Apr 1;151(4):965–968. doi: 10.1084/jem.151.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. J., Balish E. Macrophage function in germ-free, athymic (nu/nu), and conventional-flora (nu/+) mice. J Reticuloendothel Soc. 1980 Jul;28(1):55–66. [PubMed] [Google Scholar]
- Meltzer M. S. Tumoricidal responses in vitro of peritoneal macrophages from conventionally housed and germ-free nude mice. Cell Immunol. 1976 Mar 1;22(1):176–181. doi: 10.1016/0008-8749(76)90018-6. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Juangbhanich C. W., Nathan C. F., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. II. The role of oxygen intermediates. J Exp Med. 1979 Oct 1;150(4):950–964. doi: 10.1084/jem.150.4.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Brukner L. H., Silverstein S. C., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. I. Pharmacologic triggering of effector cells and the release of hydrogen peroxide. J Exp Med. 1979 Jan 1;149(1):84–99. doi: 10.1084/jem.149.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newborg M. F., North R. J. On the mechanism of T cell-independent anti-Listeria resistance in nude mice. J Immunol. 1980 Feb;124(2):571–576. [PubMed] [Google Scholar]
- Nickol A. D., Bonventre P. F. Anomalous high native resistance to athymic mice to bacterial pathogens. Infect Immun. 1977 Dec;18(3):636–645. doi: 10.1128/iai.18.3.636-645.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst M. J., Hedegaard H. B., Johnston R. B., Jr Cultured human monocytes require exposure to bacterial products to maintain an optimal oxygen radical response. J Immunol. 1982 Jan;128(1):123–128. [PubMed] [Google Scholar]
- Pace J. L., Russell S. W., Torres B. A., Johnson H. M., Gray P. W. Recombinant mouse gamma interferon induces the priming step in macrophage activation for tumor cell killing. J Immunol. 1983 May;130(5):2011–2013. [PubMed] [Google Scholar]
- Raff M. C. Theta-bearing lymphocytes in nude mice. Nature. 1973 Dec 7;246(5432):350–351. doi: 10.1038/246350a0. [DOI] [PubMed] [Google Scholar]
- Rao G. R., Rawls W. E., Perey D. Y., Tompkins W. A. Macrophage activation in congenitally athymic mice raised under conventional or germ-free conditions. J Reticuloendothel Soc. 1977 Jan;21(1):13–20. [PubMed] [Google Scholar]
- Sharma S. D., Middlebrook G. Partial purification and properties of an antibacterial product of peritoneal exudate cell cultures from BCG-infected guinea pigs. Infect Immun. 1977 Mar;15(3):737–744. doi: 10.1128/iai.15.3.737-744.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz L. D., Wilder M. S. Cytotoxicity of rabbit blood for Listeria monocytogenes. Infect Immun. 1971 Dec;4(6):703–708. doi: 10.1128/iai.4.6.703-708.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirsing von Koenig C. H., Heymer B., Finger H. Resistance of gnotobiotic nude mice to Listeria infection. Ann Immunol (Paris) 1984 Jul-Aug;135D(1):51–58. doi: 10.1016/s0769-2625(84)80154-3. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Blanden R. V. Macrophage activation in mice lacking thymus-derived (T) cells. Experientia. 1975 May 15;31(5):591–593. doi: 10.1007/BF01932477. [DOI] [PubMed] [Google Scholar]