Abstract
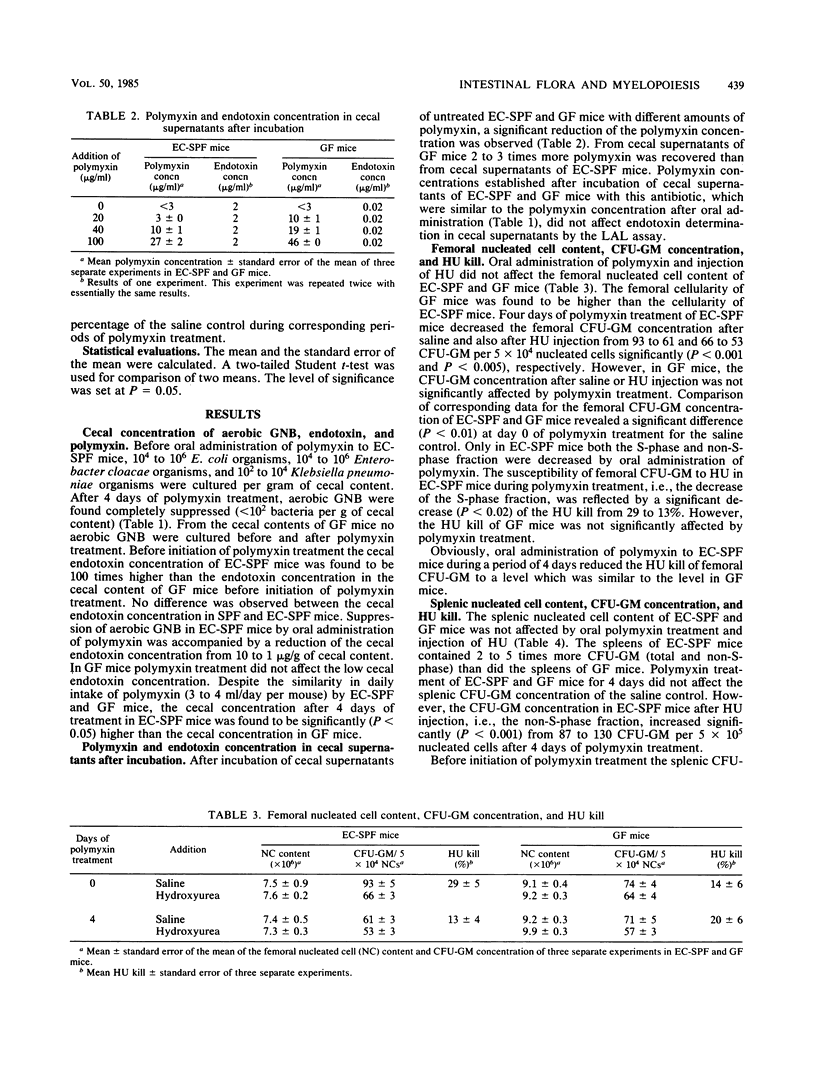
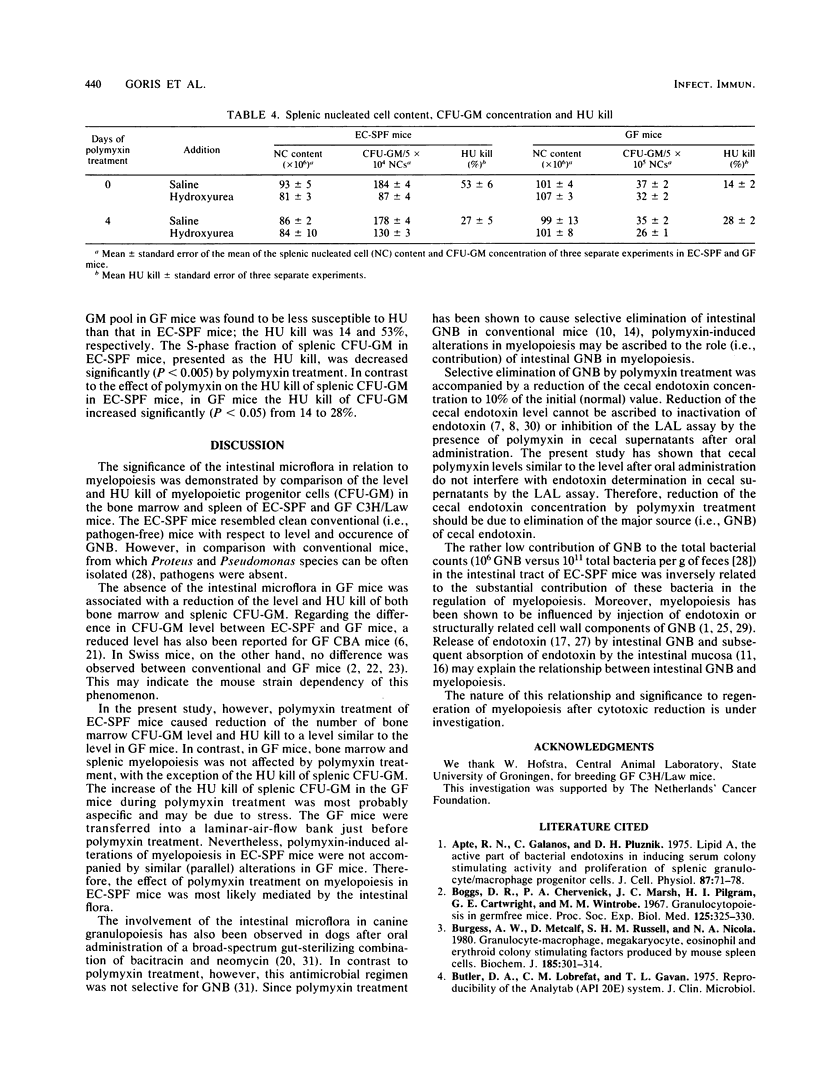
Oral administration of polymyxin to specific-pathogen-free C3H/Law mice which with previously contaminated with gram-negative bacteria resulted in complete suppression of cecal gram-negative bacteria. Suppression of cecal gram-negative bacteria was accompanied by reduction of the cecal endotoxin concentration from 10 to 1 microgram/g of cecal content as measured with a microtechnique for the Limulus amebocyte lysate assay. Endotoxin determination by this assay appeared to be unaffected by the amount of polymyxin present in cecal preparations after oral administration of this antibiotic. In experimentally contaminated specific-pathogen-free mice, the femoral concentration of progenitor cells forming granulocyte-macrophage colonies in vitro (CFU-GM) decreased significantly (P less than 0.001) to 66% of the initial control after 4 days of polymyxin treatment. However, the femoral CFU-GM concentration in germfree mice and splenic CFU-GM concentration in experimentally contaminated specific-pathogen-free and germfree mice was not affected by polymyxin treatment. The kinetic behavior of femoral and splenic CFU-GM in experimentally contaminated specific-pathogen-free and germfree mice was expressed as the in vivo sensitivity to the S-phase-specific cytostatic drug hydroxyurea, i.e., the hydroxyurea kill. Administration of polymyxin to experimentally contaminated specific-pathogen-free mice significantly diminished the hydroxyurea kill of femoral CFU-GM from 29 to 13% (P less than 0.02) and of splenic CFU-GM from 53 to 27% (P less than 0.005). The hydroxyurea kill of femoral CFU-GM in germfree mice was not significantly affected by polymyxin treatment. On basis of these results we conclude that the effect of polymyxin treatment on myelopoiesis is most likely due to elimination of intestinal gram-negative bacteria and may indicate a significant role of intestinal gram-negative bacteria in the regulation of myelopoiesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apte R. N., Galanos C., Pluznik D. H. Lipid A, the active part of bacterial endotoxins in inducing serum colony stimulating activity and proliferation of splenic granulocyte/macrophage progenitor cells. J Cell Physiol. 1976 Jan;87(1):71–78. doi: 10.1002/jcp.1040870110. [DOI] [PubMed] [Google Scholar]
- Boggs D. R., Chervenick P. A., Marsh J. C., Pilgrim H. I., Cartwright G. E., Winetrobe M. M. Granulocytopoiesis in germfree mice. Proc Soc Exp Biol Med. 1967 May;125(1):325–330. doi: 10.3181/00379727-125-32084. [DOI] [PubMed] [Google Scholar]
- Burgess A. W., Metcalf D., Russell S. H., Nicola N. A. Granulocyte/macrophage-, megakaryocyte-, eosinophil- and erythroid-colony-stimulating factors produced by mouse spleen cells. Biochem J. 1980 Feb 1;185(2):301–314. doi: 10.1042/bj1850301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caridis D. T., Reinhold R. B., Woodruff P. W., Fine J. Endotoxaemia in man. Lancet. 1972 Jun 24;1(7765):1381–1385. doi: 10.1016/s0140-6736(72)91108-7. [DOI] [PubMed] [Google Scholar]
- Chang C. F., Pollard M. Effects of microbial flora on levels of colonay stimulating factor in serums of irradiated CFW mice. Proc Soc Exp Biol Med. 1973 Oct 1;144(1):177–180. doi: 10.3181/00379727-144-37551. [DOI] [PubMed] [Google Scholar]
- Cooperstock M. S. Inactivation of endotoxin by polymyxin B. Antimicrob Agents Chemother. 1974 Oct;6(4):422–425. doi: 10.1128/aac.6.4.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooperstock M., Riegle L. Polymyxin B inactivation of lipopolysaccharide in vaccines of Gram-negative bacteria. Infect Immun. 1981 Jul;33(1):315–318. doi: 10.1128/iai.33.1.315-318.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves A. C., Bruce W. R. Endotoxin-induced sensitivity of hematopoietic stem cells to chemotherapeutic agents. Ser Haematol. 1972;5(2):64–72. [PubMed] [Google Scholar]
- Emmelot C. H., van der Waaij D. The dose at which neomycin and polymyxin B can be applied for selective decontamination of the digestive tract in mice. J Hyg (Lond) 1980 Jun;84(3):331–340. doi: 10.1017/s0022172400026851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gans H., Matsumoto K. The escape of endotoxin from the intestine. Surg Gynecol Obstet. 1974 Sep;139(3):395–402. [PubMed] [Google Scholar]
- Gardi A., Arpagaus G. R. Improved microtechnique for endotoxin assay by the Limulus amebocyte lysate test. Anal Biochem. 1980 Dec;109(2):382–385. doi: 10.1016/0003-2697(80)90664-8. [DOI] [PubMed] [Google Scholar]
- Goss W. A., Cimijotti E. B. Evaluation of an automatic diluting device for microbiological applications. Appl Microbiol. 1968 Sep;16(9):1414–1416. doi: 10.1128/am.16.9.1414-1416.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazenberg M. P., Van de Boom M., Bakker M., Van de Merwe J. P. Effect of antibiotics on the human intestinal flora in mice. Antonie Van Leeuwenhoek. 1983 Jun;49(2):97–109. doi: 10.1007/BF00393667. [DOI] [PubMed] [Google Scholar]
- Iscove N. N., Sieber F. Erythroid progenitors in mouse bone marrow detected by macroscopic colony formation in culture. Exp Hematol. 1975 Jan;3(1):32–43. [PubMed] [Google Scholar]
- Jorgensen J. H., Smith R. F. Measurement of bound and free endotoxin by the Limulus assay. Proc Soc Exp Biol Med. 1974 Sep;146(4):1024–1031. doi: 10.3181/00379727-146-38240. [DOI] [PubMed] [Google Scholar]
- Joshi J. H., Entringer M. A., Robinson W. A. Bacterial stimulation of serum colony-stimulating activity and neutrophil production in germ-free mice. Proc Soc Exp Biol Med. 1979 Oct;162(1):44–47. doi: 10.3181/00379727-162-40615. [DOI] [PubMed] [Google Scholar]
- Levin J., Poore T. E., Zauber N. P., Oser R. S. Detection of endotoxin in the blood of patients with sepsis due to gran-negative bacteria. N Engl J Med. 1970 Dec 10;283(24):1313–1316. doi: 10.1056/NEJM197012102832404. [DOI] [PubMed] [Google Scholar]
- MacVittie T. J., Walker R. I. Canine granulopoiesis: alterations induced by suppression of gram-negative flora. Exp Hematol. 1978 Sep;6(8):639–647. [PubMed] [Google Scholar]
- Metcalf D. Effect of thymidine suiciding on colony formation in vitro by mouse hematopoietic cells. Proc Soc Exp Biol Med. 1972 Feb;139(2):511–514. doi: 10.3181/00379727-139-36175. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Stevens S. Influence of age and antigenic stimulation on granulocyte and macrophage progenitor cells in the mouse spleen. Cell Tissue Kinet. 1972 Sep;5(5):433–446. doi: 10.1111/j.1365-2184.1972.tb00381.x. [DOI] [PubMed] [Google Scholar]
- Murray P. R. Standardization of the Analytab Enteric (API 20E) system to increase accuracy and reproducibility of the test for biotype characterization of bacteria. J Clin Microbiol. 1978 Jul;8(1):46–49. doi: 10.1128/jcm.8.1.46-49.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesenberry P., Morley A., Stohlman F., Jr, Rickard K., Howard D., Smith M. Effect of endotoxin on granulopoiesis and colony-stimulating factor. N Engl J Med. 1972 Feb 3;286(5):227–232. doi: 10.1056/NEJM197202032860502. [DOI] [PubMed] [Google Scholar]
- Rickard K. A., Shadduck R. K., Howard D. E., Stohlman F., Jr A differential effect of hydroxyurea on hemopoietic stem cell colonies in vitro and in vivo. Proc Soc Exp Biol Med. 1970 May;134(1):152–156. doi: 10.3181/00379727-134-34749. [DOI] [PubMed] [Google Scholar]
- Rothfield L., Pearlman-Kothencz M. Synthesis and assembly of bacterial membrane components. A lipopolysaccharide-phospholipid-protein complex excreted by living bacteria. J Mol Biol. 1969 Sep 28;44(3):477–492. doi: 10.1016/0022-2836(69)90374-x. [DOI] [PubMed] [Google Scholar]
- Savage D. C. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- Staber F. G., Tarcsay L., Dukor P. Modulations of myelopoiesis in vivo by chemically pure preparations of cell wall components from gram-negative bacteria: effects at different stages. Infect Immun. 1978 Apr;20(1):40–49. doi: 10.1128/iai.20.1.40-49.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WRIGHT W. W., WELCH H. Chemical, biological and clinical observations on colistin. Antibiot Annu. 1959;7:61–74. [PubMed] [Google Scholar]
- Walker R. I., MacVittie T. J., Sinha B. L., Ewald P. E., Egan J. E., McClung G. L. Antibiotic decontamination of the dog and its consequences. Lab Anim Sci. 1978 Feb;28(1):55–61. [PubMed] [Google Scholar]



