Abstract
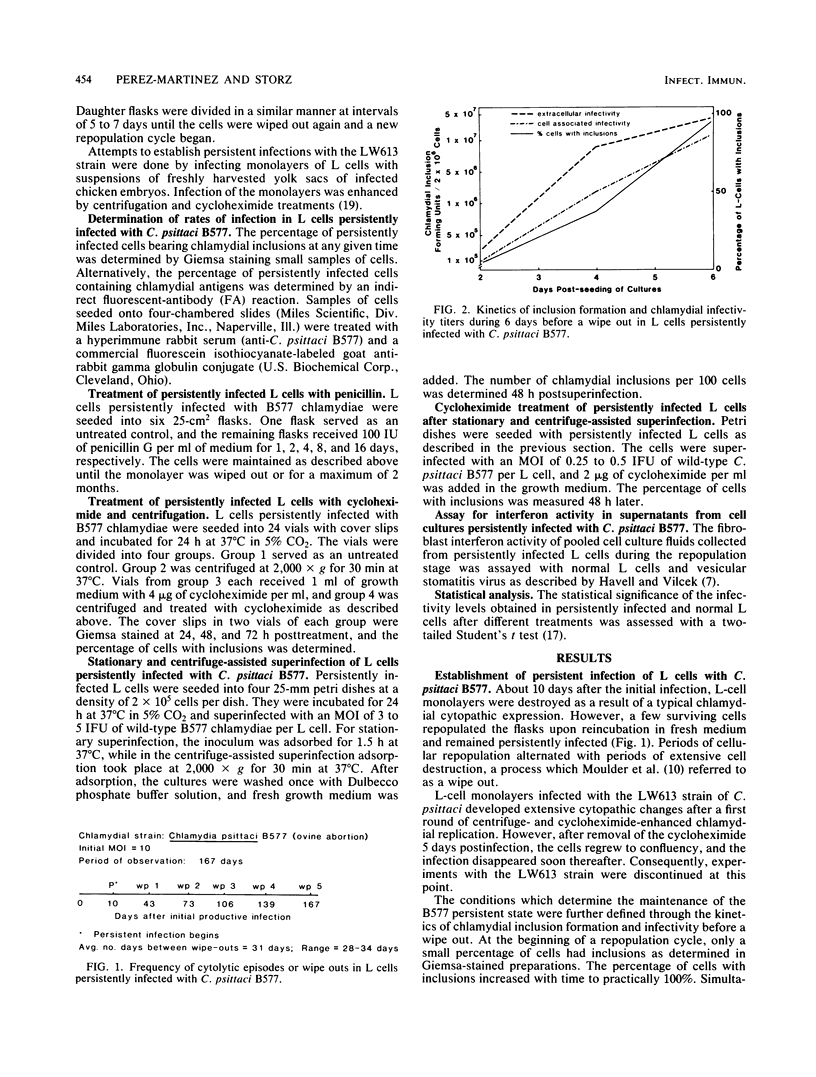
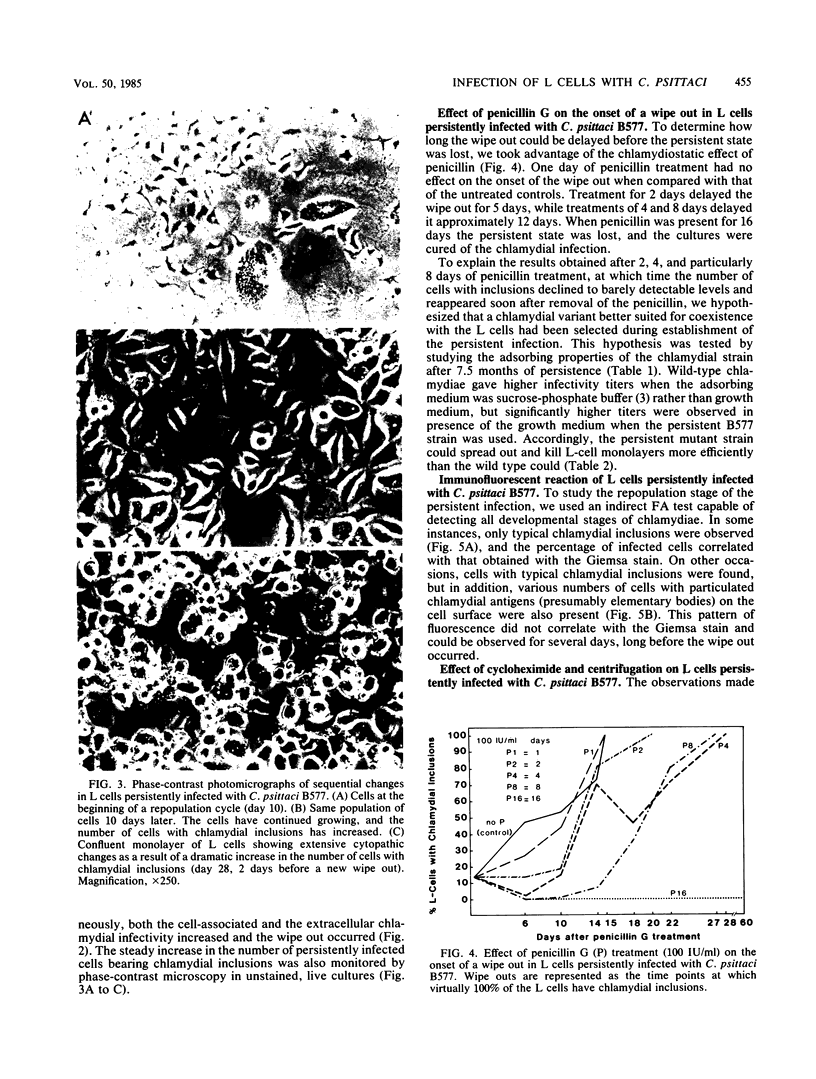
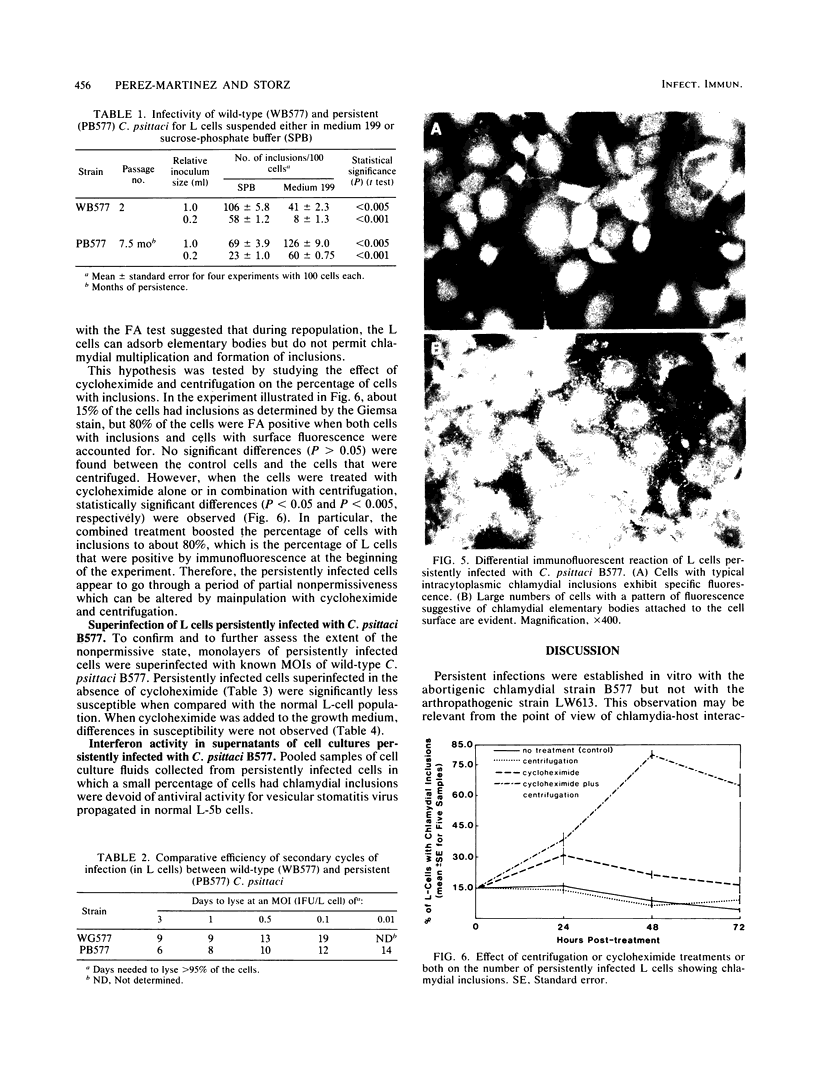
L cells inoculated at multiplicities of infection greater than or equal to 1 inclusion-forming unit of the abortigenic chlamydial strain B577 were destroyed within 10 to 15 days. Upon continued incubation in fresh medium, a few surviving cells repopulated the flasks, and the reemerging cultures remained persistently infected. The persistent state was characterized by cycles of repopulation with a low ratio of infected cells and cycles of extensive cytopathic changes in which greater than 90% of the cells had chlamydial inclusions and which could be delayed or even terminated by penicillin treatment. Immunofluorescence and superinfection during the period of repopulation revealed that the persistently infected cells could adsorb chlamydiae but their multiplication was arrested. This nonpermissive state could be terminated by the specific action of cycloheximide. L cells spontaneously cured from a persistent infection exhibited no change in susceptibility to chlamydiae when compared with normal L cells. However, chlamydiae derived from L cells after 7.5 months of persistence destroyed L-cell monolayers more rapidly and at lower multiplicities of infection than the wild type. This state of chlamydia-host cell interaction could not be established with the arthropathogenic strain LW613 because chlamydial infectivity was lost after the first cytolytic burst of infection in the cell cultures. The persistence described for the strain B577-L-cell system appears to differ from previously described models involving other chlamydial strains.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan I., Pearce J. H. Differential amino acid utilization by Chlamydia psittaci (strain guinea pig inclusion conjunctivitis) and its regulatory effect on chlamydial growth. J Gen Microbiol. 1983 Jul;129(7):1991–2000. doi: 10.1099/00221287-129-7-1991. [DOI] [PubMed] [Google Scholar]
- BADER J. P., MORGAN H. R. Latent viral infection of cells in tissue culture. VII. Role of water-soluble vitamins in psittacosis virus propagation in L cells. J Exp Med. 1961 Feb 1;113:271–281. doi: 10.1084/jem.113.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOVARNICK M. R., MILLER J. C., SNYDER J. C. The influence of certain salts, amino acids, sugars, and proteins on the stability of rickettsiae. J Bacteriol. 1950 Apr;59(4):509–522. doi: 10.1128/jb.59.4.509-522.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I., Faubion C. L. Lymphokine-mediated microbistatic mechanisms restrict Chlamydia psittaci growth in macrophages. J Immunol. 1982 Jan;128(1):469–474. [PubMed] [Google Scholar]
- Eugster A. K., Storz J. Pathogenetic events in intestinal chlamydial infections leading to polyarthritis in calves. J Infect Dis. 1971 Jan;123(1):41–50. doi: 10.1093/infdis/123.1.41. [DOI] [PubMed] [Google Scholar]
- Hatch T. P. Competition between Chlamydia psittaci and L cells for host isoleucine pools: a limiting factor in chlamydial multiplication. Infect Immun. 1975 Jul;12(1):211–220. doi: 10.1128/iai.12.1.211-220.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell E. A., Vilcek J. Production of high-titered interferon in cultures of human diploid cells. Antimicrob Agents Chemother. 1972 Dec;2(6):476–484. doi: 10.1128/aac.2.6.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. K., Moulder J. W. Persistent infection of mouse fibroblasts (McCoy cells) with a trachoma strain of Chlamydia trachomatis. Infect Immun. 1981 May;32(2):822–829. doi: 10.1128/iai.32.2.822-829.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. Inhibition of onset of overt multiplication of Chlamydia psittaci in persistently infected mouse fibroblasts (L cells). Infect Immun. 1983 Feb;39(2):898–907. doi: 10.1128/iai.39.2.898-907.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W., Levy N. J., Schulman L. P. Persistent infection of mouse fibroblasts (L cells) with Chlamydia psittaci: evidence for a cryptic chlamydial form. Infect Immun. 1980 Dec;30(3):874–883. doi: 10.1128/iai.30.3.874-883.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W., Levy N. J., Zeichner S. L., Lee C. K. Attachment defect in mouse fibroblasts (L cells) persistently infected with Chlamydia psittaci. Infect Immun. 1981 Oct;34(1):285–291. doi: 10.1128/iai.34.1.285-291.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanne G. Infection chlamydiale persistante non apparente, dans des cellules de McCoy. Rev Can Biol. 1981 Jun;40(2):195–201. [PubMed] [Google Scholar]
- Page L. A., Matthews P. J., Smith P. C. Natural intestinal infection with Chlamydia psittaci in a closed bovine herd: serologic changes, incidence of shedding, antibiotic treatment of the herd, and biologic characteristics of the Chlamydiae. Am J Vet Res. 1973 May;34(5):611–614. [PubMed] [Google Scholar]
- Rothermel C. D., Byrne G. I., Havell E. A. Effect of interferon on the growth of Chlamydia trachomatis in mouse fibroblasts (L cells). Infect Immun. 1983 Jan;39(1):362–370. doi: 10.1128/iai.39.1.362-370.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rønsholt L. Chlamydia psittaci infection in Danish cattle. Acta Pathol Microbiol Scand B. 1978 Oct;86B(5):291–297. doi: 10.1111/j.1699-0463.1978.tb00046.x. [DOI] [PubMed] [Google Scholar]
- STORZ J. SUPERINFECTION OF PREGNANT EWES LATENTLY INFECTED WITH A PSITTACOSIS-LYMPHOGRANULOMA AGENT. Cornell Vet. 1963 Oct;53:469–480. [PubMed] [Google Scholar]
- Smith P. C., Cutlip R. C., Page L. A. Pathogenicity of a strain of Chlamydia psittaci of bovine intestinal origin for neonatal calves. Am J Vet Res. 1973 May;34(5):615–618. [PubMed] [Google Scholar]
- Spears P., Storz J. Biotyping of Chlamydia psittaci based on inclusion morphology and response to diethylaminoethyl-dextran and cycloheximide. Infect Immun. 1979 Apr;24(1):224–232. doi: 10.1128/iai.24.1.224-232.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spears P., Storz J. Chlamydia psittaci: growth characteristics and enumeration of serotypes 1 and 2 in cultured cells. J Infect Dis. 1979 Dec;140(6):959–967. doi: 10.1093/infdis/140.6.959. [DOI] [PubMed] [Google Scholar]
- Storz J. Psittacosis-lymphogranuloma infection of sheep. Antigenic structures and interrelations of PL agents associated with polyarthritis, enzootic abortion, intrauterine and latent intestinal infections. J Comp Pathol. 1966 Oct;76(4):351–362. doi: 10.1016/0021-9975(66)90055-7. [DOI] [PubMed] [Google Scholar]
- Storz J., Smart R. A., Marriott M. E., Davis R. V. Polyarthritis of calves: isolation of psittacosis agents from affected joints. Am J Vet Res. 1966 May;27(118):633–641. [PubMed] [Google Scholar]