Abstract
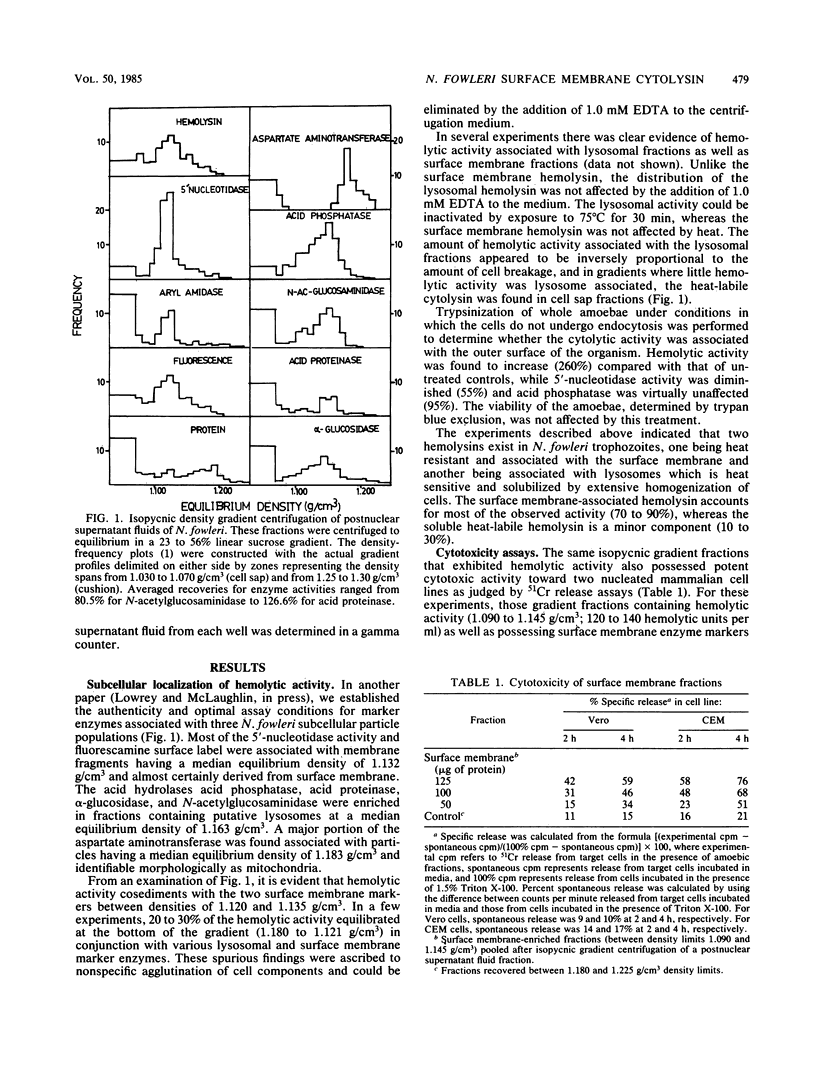
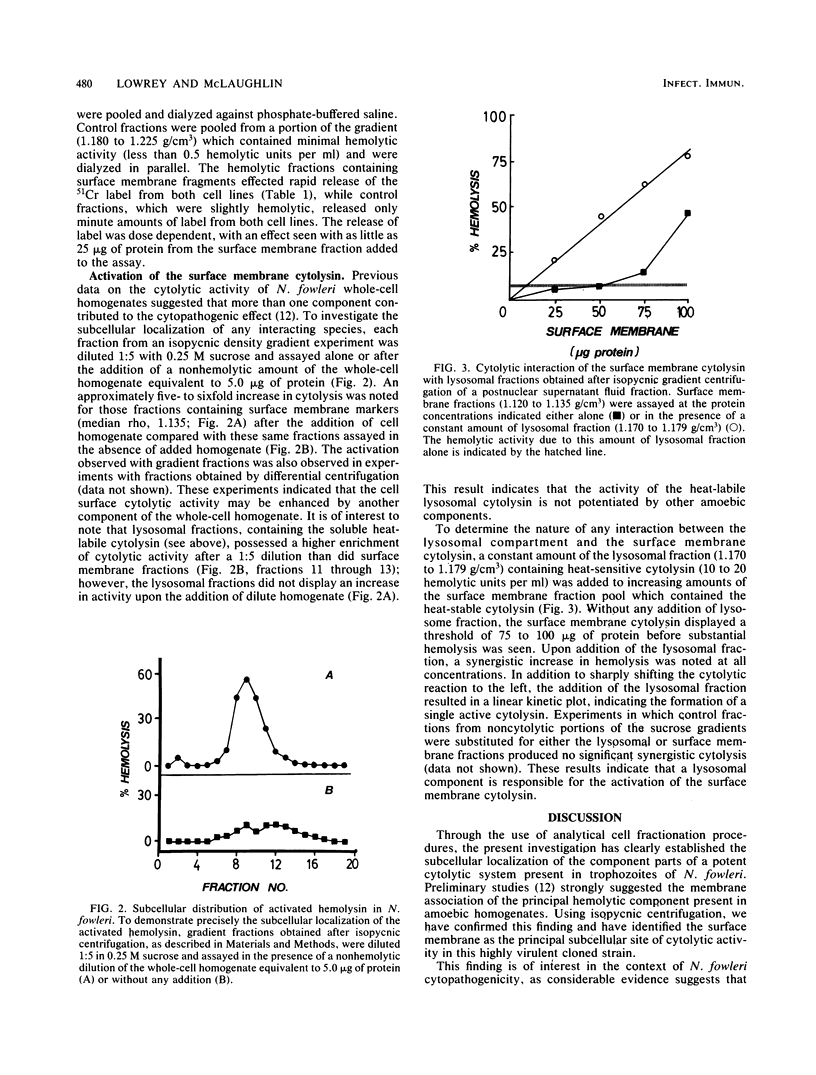
Surface membrane-enriched fractions of Naegleria fowleri obtained after isopycnic centrifugation experiments contain a potent cytolytic activity as determined by hemolysis and 51Cr release assays. This surface membrane cytolysin was unaffected by a treatment at 75 degrees C for 30 min and accounted for 70 to 90% of cytolysis by whole-cell lysates of amoebae. This heat resistance as well as intimate membrane association distinguished the surface membrane cytolytic activity from a second heat-labile cytolytic activity which appears to be latent within lysosomes. The surface membrane cytolysin was found to be specifically activated by diluted samples of lysosomal fractions. The possible role of this surface membrane cytotoxin in the pathogenicity of N. fowleri is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bostian K. A., Jayachandran S., Tipper D. J. A glycosylated protoxin in killer yeast: models for its structure and maturation. Cell. 1983 Jan;32(1):169–180. doi: 10.1016/0092-8674(83)90507-x. [DOI] [PubMed] [Google Scholar]
- Brown T. Inhibition by amoeba-specific antiserum and by cytochalasin B of the cytopathogenicity of Naegleria fowleri in mouse embryo-cell cultures. J Med Microbiol. 1979 Aug;12(3):355–362. doi: 10.1099/00222615-12-3-355. [DOI] [PubMed] [Google Scholar]
- Brown T. Observations by light microscopy on the cytopathogenicity of Naegleria fowleri in mouse embryo-cell cultures. J Med Microbiol. 1978 Aug;11(3):249–259. doi: 10.1099/00222615-11-3-249. [DOI] [PubMed] [Google Scholar]
- Dunnebacke T. H., Schuster F. L. The nature of a cytopathogenic material present in amebae of the genus Naegleria. Am J Trop Med Hyg. 1977 May;26(3):412–421. doi: 10.4269/ajtmh.1977.26.412. [DOI] [PubMed] [Google Scholar]
- FOLEY G. E., LAZARUS H., FARBER S., UZMAN B. G., BOONE B. A., MCCARTHY R. E. CONTINUOUS CULTURE OF HUMAN LYMPHOBLASTS FROM PERIPHERAL BLOOD OF A CHILD WITH ACUTE LEUKEMIA. Cancer. 1965 Apr;18:522–529. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Fulford D. E., Bradley S. G., Marciano-Cabral F. Cytopathogenicity of Naegleria fowleri for cultured rat neuroblastoma cells. J Protozool. 1985 Feb;32(1):176–180. doi: 10.1111/j.1550-7408.1985.tb03034.x. [DOI] [PubMed] [Google Scholar]
- Henkart P. A., Millard P. J., Reynolds C. W., Henkart M. P. Cytolytic activity of purified cytoplasmic granules from cytotoxic rat large granular lymphocyte tumors. J Exp Med. 1984 Jul 1;160(1):75–93. doi: 10.1084/jem.160.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysmith R. M., Franson R. C. Degradation of human myelin phospholipids by phospholipase-enriched culture media of pathogenic Naegleria fowleri. Biochim Biophys Acta. 1982 Sep 14;712(3):698–701. doi: 10.1016/0005-2760(82)90300-9. [DOI] [PubMed] [Google Scholar]
- John D. T., Cole T. B., Jr, Marciano-Cabral F. M. Sucker-like structures on the pathogenic amoeba Naegleria fowleri. Appl Environ Microbiol. 1984 Jan;47(1):12–14. doi: 10.1128/aem.47.1.12-14.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John D. T. Primary amebic meningoencephalitis and the biology of Naegleria fowleri. Annu Rev Microbiol. 1982;36:101–123. doi: 10.1146/annurev.mi.36.100182.000533. [DOI] [PubMed] [Google Scholar]
- Lowrey D. M., McLaughlin J. A multicomponent hemolytic system in the pathogenic amoeba Naegleria fowleri. Infect Immun. 1984 Sep;45(3):731–736. doi: 10.1128/iai.45.3.731-736.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrey D. M., McLaughlin J. A simple and reliable method for cloning Naegleria fowleri. J Parasitol. 1984 Dec;70(6):991–992. [PubMed] [Google Scholar]
- Marciano-Cabral F. M., Patterson M., John D. T., Bradley S. G. Cytopathogenicity of Naegleria fowleri and Naegleria gruberi for established mammalian cell cultures. J Parasitol. 1982 Dec;68(6):1110–1116. [PubMed] [Google Scholar]
- Müller M. Biochemical cytology of trichomonad flagellates. I. Subcellular localization of hydrolases, dehydrogenases, and catalase in Tritrichomonas foetus. J Cell Biol. 1973 May;57(2):453–474. doi: 10.1083/jcb.57.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page F. C. Taxonomic criteria for limax amoebae, with descriptions of 3 new species of Hartmannella and 3 of Vahlkampfia. J Protozool. 1967 Aug;14(3):499–521. doi: 10.1111/j.1550-7408.1967.tb02036.x. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Konigsberg P. J. Cytolytic T cell granules. Isolation, structural, biochemical, and functional characterization. J Exp Med. 1984 Sep 1;160(3):695–710. doi: 10.1084/jem.160.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravdin J. I., Croft B. Y., Guerrant R. L. Cytopathogenic mechanisms of Entamoeba histolytica. J Exp Med. 1980 Aug 1;152(2):377–390. doi: 10.1084/jem.152.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weik R. R., John D. T. Agitated mass cultivation of Naegleria fowleri. J Parasitol. 1977 Oct;63(5):868–871. [PubMed] [Google Scholar]
- Wong M. M., Karr S. L., Jr, Chow C. K. Changes in the virulence of Naegleria fowleri maintained in vitro. J Parasitol. 1977 Oct;63(5):872–878. [PubMed] [Google Scholar]
- Zusman N., Cafmeyer N., Hudson R. A. Use of erythrocyte hemolysis kinetics in the purification of complex cardiotoxin mixtures. Toxicon. 1982;20(2):517–520. doi: 10.1016/0041-0101(82)90018-6. [DOI] [PubMed] [Google Scholar]



