Abstract
The vitronectin receptor is a member of the integrin family of adhesion protein receptors and binds a broad spectrum of ligands, including fibronectin and fibrinogen in addition to vitronectin. We have generated four mAbs that recognize the murine αvβ3 vitronectin receptor. Biochemical and expression analyses showed that two of the mAbs are specific for the αv chain, and two are specific for the β3 chain. The mAbs are effective blocking reagents and inhibited cell adhesion to vitronectin, fibrinogen, and fibronectin. Staining analysis revealed expression of αv and β3 on certain populations of murine thymocytes, splenocytes, and bone marrow cells. The expression of αv and β3 appeared to be modulated at specific stages of thymocyte development, suggesting a possible function for the αvβ3 vitronectin receptor in T cell development.
Keywords: monoclonal antibodies, thymocyte development
Integrins comprise a large family of heterodimeric cell surface proteins composed of α and β chains that mediate cell–cell interactions and interactions between cells and the extracellular matrix (ECM) (1–3). Integrins are expressed on all cells and are involved in a number of fundamental cell processes including adhesion, migration, activation, and differentiation. The expression and functions of integrins have been particularly well-studied with respect to lymphocytes, which require a variety of cell–cell and cell–ECM interactions to perform their complex programs of immune surveillance and antigen response (4, 5). The functions of integrins depend on binding to specific adhesion proteins, such as fibronectin, often through recognition of the tripeptide, arginine-glycine-aspartic acid (RGD), binding motif (6).
The human αvβ3 vitronectin receptor was originally identified as a heterodimeric molecule with vitronectin binding activity (7), and later shown to be related to other members of the integrin family (8). Subsequent studies demonstrated that this receptor has a broad binding specificity and can mediate binding to fibronectin, fibrinogen, von Willebrand factor, and thrombospondin in addition to vitronectin (9–12). More recently, a mAb specific for the murine αvβ3 vitronectin receptor (αvβ3) was isolated (13, 14), and used to identify αvβ3 as a costimulatory molecule required for spontaneous activation of γδ T cell hybridomas (15).
In the process of identifying cell surface molecules involved in the constitutive interleukin 2 (IL-2) secretion of murine Vγ1, Vδ6 T cell hybridomas, we generated a series of monoclonal antibodies (mAbs) that recognize murine αvβ3. Here, we present the characterization of four of these mAbs, including the determination of their chain specificities and staining analysis of several lymphoid populations. Contrary to previous reports (13, 16), we observed detectable levels of αv and β3 expression on certain populations of thymocytes and splenocytes in addition to bone marrow cells. Interestingly, we observed differential expression of the αv and β3 chains on discrete populations of thymocytes, suggesting a possible role for this receptor in T cell development.
MATERIALS AND METHODS
Animals.
C57BL/6 (B6) mice were obtained from Taconic Farms or from Iffa Credo. Rag-1-deficient mice were as described (17). Adult Armenian hamsters were obtained from Cytogen (West Roxbury, MA).
Antibodies and Antisera.
The mAbs used were 145-2C11, anti-CD3, RM4-5, anti-CD4, 53-6.7, anti-CD8α, RA3-6B2, anti-B220, and 2C9.G2, anti-CD61. These mAbs were purchased as fluorescein isothiocyanate (FITC) or biotin conjugates from PharMingen. The anti-β1 antiserum was as described (18). The anti-β3 antiserum, 8275, was a gift from Dr. Mark Ginsberg (Scripps Research Institute). The anti-αv antiserum was purchased from Chemicon.
Production and Purification of the Anti-Vitronectin Receptor mAbs.
The production of mAbs capable of inhibiting the constitutive IL-2 production by Vγ1, Vδ6-expressing γδ T cell hybridomas has been described in detail (19). 8-B11 is a hamster IgG and was purified from tissue culture supernatant by protein G affinity chromatography. The other three mAbs (8-2D, 8-B3, and 4-10D) are of the IgM class, and were partially purified from tissue culture supernatant. Briefly, cells were adapted to grow in either Hybridoma-SFM (GIBCO/BRL) or Optimem (GIBCO/BRL) in the absence of serum, and the collected supernatants were extensively filtered through a 100-kDa pore-size membrane (YM100, Amicon). Purified mAbs were conjugated with FITC or biotin according to standard procedures.
Cell Surface Labeling and Immunoprecipitation.
As shown in Fig. 1 A and B, 107 cells were harvested by centrifugation, washed three times with PBS, resuspended in 200 μl of PBS, and labeled with 1 mCi (1 Ci = 37 GBq) of Na125I by the lactoperoxidase method. After three washes, the cell pellet was lysed in 1 ml of ice-cold lysis buffer (10 mM Tris·HCl, pH 7.6/150 mM NaCl/1% Triton X-100/1% BSA/1 mM phenylmethylsulfonyl fluoride), vortexed for 20 s, and incubated on ice for 30 min. The lysate was clarified by centrifugation at 13,000 rpm at 4°C in a microfuge. Normal hamster serum (100 μl) was added to the supernatant followed by two rounds of preclearing with one-tenth volume protein A-Sepharose beads (Pharmacia). One quarter of the precleared lysate was incubated with 10 μg of biotinylated mAb followed by incubation with one-tenth volume of Streptavidin-Sepharose beads. Immunoprecipitates were washed, resuspended in sample buffer, and subjected to SDS/PAGE on a 7–10% mini-gel according to standard procedures. Radioactive bands were visualized using x-ray film. The derivation and culture of embryonic day 9 (E9) fibroblasts was as described (20) (see Fig. 1C). One 6-cm plate of ≈60% confluent E9 fibroblasts was labeled as a monolayer with 1 mCi Na125I, and roughly 70 million hybridoma cells were labeled in suspension with 2 mCi Na125I by the lactoperoxidase-glucose oxidase method (21). Labeled E9 and hybridoma cells were washed extensively and lysed in 1 ml and 2 ml, respectively, of ice-cold lysis buffer (50 mM Tris HCl, pH 8.0/150 mM NaCl/0.5 mM CaCl2/0.5% Nonidet P-40/1 mM phenylmethylsulfonyl fluoride/0.02 mg/ml aprotinin/0.0125 mg/ml leupeptin). The lysates were clarified by centrifugation at 13,000 rpm at 4°C in a microfuge. Each clarified lysate (135 μl) was separated for precipitation with the anti-αv serum, and the remaining 1 ml of the E9 lysate was mixed with 1 ml of the hybridoma lysate. The mixed lysate was split into an 800-μl fraction for precipitation with the antisera and a 1200-μl fraction for precipitation with the mAbs. Samples to be precipitated with the antisera were precleared with 25% vol protein A-Sepharose, and samples to be precipitated with the biotinylated mAbs were incubated with biotinylated RM4-5 and anti-CD4 (PharMingen) and were precleared with 25% volume Ultralink-immobilized streptavidin (Pierce). The precleared 135-μl portions of the original lysates and 270-μl portions of the mixed lysate were mixed with 100 μl of 10 mg/ml BSA followed by addition of either 2–5 μl of αv, β1, or β3 antiserum, or 5–10 μg of the biotinylated anti-αvβ3 mAbs as indicated. After 2 hr incubation at 4°C, 50 μl protein A-Sepharose beads (50% slurry precoated with 10 mg/ml BSA in lysis buffer) was added for the antiserum precipitations and 50 μl Ultralink-immobilized streptavidin (50% gel precoated with 10 mg/ml BSA in lysis buffer) was added for the biotin-mAb precipitations. After 2 hr rotation at 4°C, the beads were sedimented and washed 4× with cold lysis buffer. Samples were then boiled in 50 μl of nonreducing SDS/PAGE sample buffer, and subjected to SDS/PAGE on a 6% gel.
Figure 1.
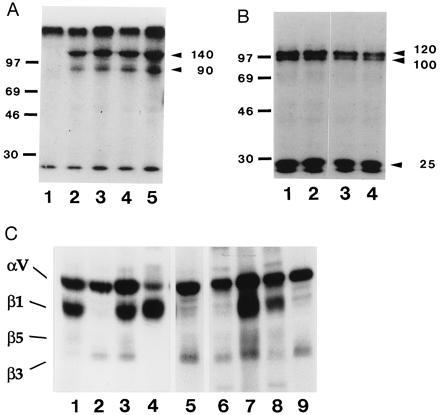
Immunoprecipitation analysis with the 8-2D, 8-B3, 4-10D, and 8-B11 mAbs. (A) SDS/PAGE analysis of immunopreciptates from lysate of 125I-labeled hybridoma cells run under nonreduced conditions. Lanes: 1, beads alone; 2, 4-10D; 3, 8-B11; 4, 8-2D; 5, 8-B3. Molecular weight standards are indicated in kilodaltons. (B) SDS/PAGE analysis of immunoprecipitates from lysate of 125I-labeled hybridoma cells run under reduced conditions. Lanes: 1, 4-10D; 2, 8-B11; 3, 8-2D; 4, 8-B3. Molecular weight standards are indicated in kilodaltons. (C) SDS/PAGE analysis of immunoprecipitates from lysates of 125I-labeled hybridoma cells and E9 fibroblasts and mixed lysate, run under nonreduced conditions. Lanes: 1, E9 fibroblast lysate, anti-αv serum; 2, hybridoma lysate, anti-αv serum; 3–9, mixed lysate precipitated with: 3, anti-αv serum; 4, anti-β1 serum; 5, anti-β3 serum; 6, 8-2D; 7, 8-B3; 8, 4-10D; 9, 8-B11. Lanes 1–4 were exposed for a shorter time than lanes 5–9, because the anti-αv and anti-β1 sera immunoprecipitate much more efficiently than the other antibodies. T3, hybridoma cells; E9, embryonic d9 fibroblasts; M, mixed lysate.
Immunofluorescence Staining and Flow Cytometric Analysis.
Cells (5 × 105) were incubated for 1 hr at 4°C in 25 μl PBS staining buffer (2% FCS/0.1% NaN3) with 20 μg/ml Fc Block (PharMingen), followed by addition of 25 μl of 40% normal hamster serum (NHS) in staining buffer. After 30 min, the cells were spun down and resuspended in staining buffer with the indicated labeled Abs for 40 min at 4°C. After two washes, the cells were incubated with streptavidin-phycoerythrin (PharMingen or Southern Biotechnology Associates) for 15 min at 4°C. After two additional washes, viable cells were analyzed using a FACScan flow cytometer (Becton Dickinson). Dead cells were gated out by staining with propidium iodide. Normal mouse serum was substituted for NHS in the staining of Rag1-deficient thymocytes.
For competition experiments, the blocking with Fc block and NHS was omitted. Cells were first incubated with a 10-fold excess of the indicated unlabeled antibodies for 15 min, before the addition of the biotinylated mAbs. The rest of the staining and analysis was performed as indicated above.
Adhesion Assay.
Preparations of mouse vitronectin (Telios Pharmaceuticals, San Diego), mouse fibronectin (GIBCO/BRL), mouse fibrinogen (Sigma), rat collagen type I, mouse collagen type IV (Sigma), or mouse laminin (ICN) were diluted in PBS and distributed in 96-well flat-bottom tissue culture plates (Costar). After an overnight incubation at 4°C, the coated wells were washed twice with PBS and incubated for 2 hr at 37°C with 100 μl of adhesion medium (RPMI medium 1640/0.2% BSA). Cells were incubated for 5 min with PBS-EDTA (1 mM), washed three times with adhesion medium, and resuspended at a concentration of 5 × 105 cells per ml. The cell suspension (100 μl) was added to the saturated wells, and, after a 10 sec centrifugation at 200 g, the plates were incubated at 37°C for 2 hr. For the inhibition assays, the cells were preincubated with the indicated concentrations of antibody or peptide (GRGDSP or GRGESP, Sigma) for 45 min at 4°C in 96-well plates in a final volume of 100 μl before being transfered to the coated plates. Nonadherent cells were removed from the wells by the careful addition and removal of 200 μl of adhesion medium, which was repeated three times. Finally, 200 μl of RPMI containing 5% FCS and 10 μg MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, Sigma] was added to each well and the plates were incubated at 37°C for 3 hr. The plates were centrifuged for 3 min at 200 × g, and the supernatants were flicked out from the plates. Dimethyl sulfoxide (200 μl) was then added to the wells and pipetted up and down until the precipitated salt was dissolved; the OD540 was then read. All conditions were performed in duplicate. Percent adherence is defined as the OD of the coated wells divided by the OD of wells that had the same number of input cells and were not washed. The linear correlation between the number of cells in the wells and the OD was checked in every experiment.
RESULTS
Characterization of Anti-Vitronectin Receptor mAbs.
The mAbs 8-2D, 8-B3, 4-10D, and 8-B11 were identified based on their ability to block spontaneous IL-2 secretion of murine Vγ1, Vδ6-expressing γδ T-cell hybridomas. SDS/PAGE analysis of immunoprecipitations with each of the four antibodies from lysates of 125I-surface-labeled hybridoma cells under nonreduced (Fig. 1A) and reduced (Fig. 1B) conditions revealed the characteristic pattern of bands for αvβ3. Under nonreduced conditions, the band of approximately 140 kDa corresponds to the αv chain and the band of approximately 90 kDa corresponds to the β3 chain. Under reduced conditions, the bands of approximately 120 kDa and 25 kDa correspond to the two subunits of the αv chain, and the band of approximately 100 kDa corresponds to the β3 chain. The size of the β3 chain appears smaller under nonreduced conditions due to the presence of intrachain disulfide bonds (22). To further define the specificity of the four mAbs, immunoprecipitations were performed with the four mAbs and anti-αv and anti-β3 antisera on mixed lysates from 125I-surface-labeled embryonic fibroblast cells and hybridoma cells. As shown in Fig. 1C, the embryonic cells express the αv chain primarily in combination with β1, and, to a considerably lesser extent, in combination with β3 and β5, while the hybridoma cells express the αv chain primarily in combination with the β3 chain. The mAbs 8-B3 and 4-10D precipitated αv in combination with β1, β3, and β5 from mixed lysate, while the mAbs 8-2D and 8-B11 precipitated αv only in combination with β3 (Fig. 1C). This result suggests that 8-B3 and 4-10D bind specifically to the αv chain and 8-2D and 8-B11 bind either to the β3 chain or to combined epitopes of the αvβ3 heterodimer.
To confirm the epitope specificities suggested by the immunoprecipitation analysis, binding competition assays were performed with the four mAbs and a previously characterized anti-β3 mAb, 2C9.G2. As summarized in Table 1, the five antibodies could be divided into two groups based on their ability to compete with each other for binding. 8-B3 and 4-10D effectively competed with each other for binding and failed to compete with 8-2D, 8-B11, and 2C9.G2 for binding. Accordingly, 8-2D, 8-B11, and 2C9.G2 competed with each other for binding and failed to compete with 8-B3 and 4-10D for binding. These results are in agreement with the specificities assigned based on the immunoprecipitation data.
Table 1.
Binding competition
| 8-B3 | 4-10D | 8-2D | 8-B11 | 2C9.G2 | |
|---|---|---|---|---|---|
| 8-B3 | + | + | − | − | − |
| 4-10D | + | + | − | − | − |
| 8-2D | − | − | + | + | + |
| 8-B11 | − | − | + | + | + |
| 2C9.G2 | − | − | + | + | + |
Binding competition analysis was performed as described. Cells were preincubated with the mAbs listed in the far left column and were then stained with the mAbs listed in the top row. +, Competition for binding; −, lack of competition for binding.
To further delineate the specificities of the four mAbs, resting platelets were stained and analyzed by flow cytometry. Resting platelets are known to express the β3 chain as part of the fibrinogen receptor and to express much lower levels of the αv chain (23, 24). As shown in Fig. 2, resting platelets were negative for staining with the 8-B3 and 4-10D mAbs, and were positive for staining with the 8-2D and 8-B11 mAbs. Taken together, the immunoprecipitation results, binding competition data, and platelet staining analyses strongly suggest that 8-B3 and 4-10D specifically bind to the αv chain and that 8-2D and 8-B11 specifically bind to the β3 chain.
Figure 2.
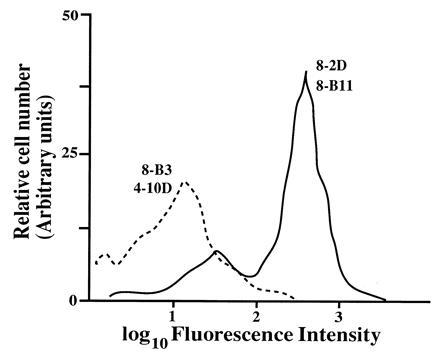
FACS analysis of platelets with the anti-αvβ3 mAbs. Platelets were diluted in Tyrode buffer (5 mM Hepes/150 mM NaCl/2.5 mM KCl/5.5 mM glucose/0.1% BSA) and incubated with the indicated FITC-labeled antibodies for 30 min on ice. The cells were further diluted and analyzed with a FACScan without washing to avoid aggregation. The fluorescence intensity profile of cells stained with the 8-B3 and 8-2D mAbs is shown. The profile observed with the 8-B3 mAb was similar to that observed with 4-10D and with that of an anti-δTCR mAb used as a negative control (not shown). Similarly, the profiles obtained with the 8-2D and 8-B11 mAbs were almost indistinguishable.
Inhibition of Substrate Binding.
The anti-vitronectin receptor antibodies were selected by a functional assay and therefore it was of interest to determine which receptor-ligand interactions are inhibited by antibody binding. As shown in Fig. 3A, BW5147 cells adhere strongly to vitronectin and, to a lesser degree, to fibronectin and fibrinogen. Cell adhesion to vitronectin was specifically inhibited by addition of 8-B3, 4-10D, 8-2D, or 8-B11 antibodies and by addition of RGD peptide (Fig. 3 B and C). Similar results were obtained for fibronectin and fibrinogen binding (data not shown), indicating that the spectrum of ligand binding that is inhibited by the αv and β3 antibodies matches the previously characterized broad binding specificity of the αvβ3 vitronectin receptor.
Figure 3.
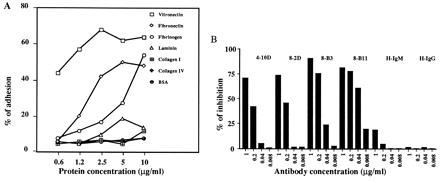
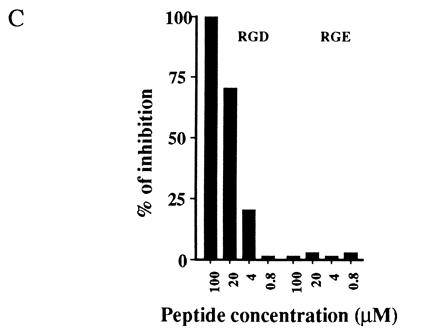
Inhibition of cell adhesion to vitronectin by anti-αvβ3 mAbs. (A) Adhesion of BW5147 cells to ECM-proteins. BW5147 cells (5 × 104 per well) were incubated in wells previously coated with increasing concentrations of the indicated ECM-proteins. The wells were washed and the remaining cells incubated with MTT. Percent adherence was calculated as described. (B and C) Inhibition of adhesion to vitronectin by anti-αvβ3 mAbs (B) and GRGDSP and GRGESP peptides (C). BW5147 cells were assayed for adhesion to wells coated with vitronectin (1 μg/ml) as described for A, after preincubation with and in the continuous presence of the indicated mAbs or peptides. H-IgM and H-IgG denote irrelevant hamster IgM and IgG mAbs, respectively. One of three independent experiments is shown. Similar results were obtained with wells coated with fibronectin (5 μg/ml) or fibrinogen (10 μg/ml) and when a Vγ1-expressing hybridoma was used instead of the BW5147 cell line.
Expression of αv and β3 Chains on Primary Lymphocytes.
To examine the expression of the αv and β3 chains on lymphocytes in vivo, cell preparations from thymus, spleen, and bone marrow of 8-week-old C57BL/6 mice were stained with the 8-B3 and 8-B11 mAbs in combination with a variety of other mAbs and analyzed by flow cytometry. In thymus, CD3+ cells were positive for staining with both 8-B3 and 8-B11, while the majority of CD3− cells exhibited significantly lower staining with both mAbs (Fig. 4A), suggesting that in the thymus, αvβ3 is primarily expressed on mature thymocytes. In spleen, CD3+ T cells were stained by both 8-B3 and 8-B11, and there appeared to be differential expression of αvβ3 on CD4+ and CD8+ cells as CD4+ cells stained more brightly than CD8+ cells with both mAbs (Fig. 4 B–D).
Figure 4.
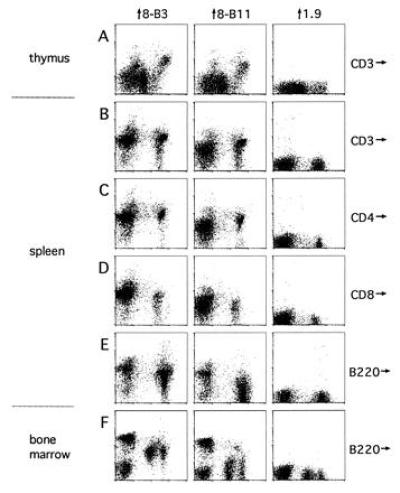
Expression of αvβ3 on different lymphoid populations. Thymus (A), spleen (B–E), and bone marrow cells (F) were stained with the indicated antibodies as described and analyzed with a FACScan. The 8-B3, 8-B11, and 1.9 mAbs were used as biotin conjugates and their binding was detected with streptavidin-phycoerythrin (vertical axes). The CD3 (A and B), CD4 (C), CD8 (D), and B220 mAbs (E and F) were used as FITC conjugates (horizontal axes). 1.9 is a hamster IgG anti-γδ TCR clonotype mAb used as a negative control. Consistent results were obtained with the 4-10D, 8-B11, and 2C9.G2 mAbs, although staining levels of some populations such as the CD3− thymocytes were higher with 4-10D than 8-B11.
In bone marrow, B220− cells could be divided into two populations based on staining with 8-B3 and 8-B11 (Fig. 4F). The 8-B3 and 8-B11 positive population appears to be precursors of granulocyte/monocyte lineage, and the 8-B3 and 8-B11 negative population appears to be erythroid precursors based on forward and side scatter properties of the cells (data not shown). The B220low pre-B cells and the B220high B cells in the bone marrow were positive for staining with 8-B3 and exhibited detectable staining on some cells with 8B11 compared with negative control mAbs (Fig. 4F). A similar staining profile was observed for B220+ B cells in the spleen (Fig. 4E).
Stainings of each of the above cell populations were performed in parallel with 4-10D, 8-2D, and 2C9.G2, and consistent results were obtained (data not shown).
Expression of αv and β3 Chains on CD4−CD8− Thymocytes.
In the stainings of the thymus, there appeared to be a small population of CD3− cells that were positive for staining with 8-B3 and 8-B11. Since CD3+ thymocytes were positive for αv and β3 expression, and CD3− thymocytes, which consist largely of the CD4+CD8+ double positive population, were low for αv and β3 expression, it was of interest to determine whether this small population of CD3−, αvβ3+ cells consists of the CD4−CD8− thymocytes, which could express αv and β3 before their development into CD4+CD8+ cells. For this purpose, thymocytes from Rag1-deficient mice, in which T cell development is blocked at the CD4−CD8− stage (17), were stained with 8-B3 and 8-B11. As shown in Fig. 5, immature thymocytes from Rag1− mice were positive for staining with both 8-B3 and 8-B11, indicating that αvβ3 is expressed at the CD4−CD8− stage of T cell development. Thus, a modulation of expression of αvβ3 appears to occur in T cell development, with CD4−CD8− cells expressing high levels of αv and β3, CD4+CD8+ cells expressing much reduced levels of αv and β3, and mature CD3+ cells expressing significant levels of αv and β3.
Figure 5.
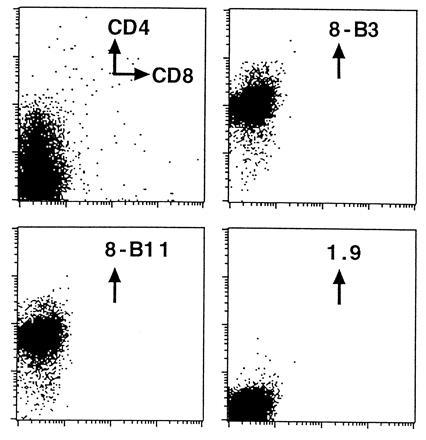
Expression of αvβ3 on CD4−CD8− thymocytes. Thymocytes from Rag1-deficient mice were stained with the indicated antibodies as described. The 8-B3, 8-B11, and 1.9 mAbs were used as biotin conjugates and their binding was detected with streptavidin-phycoerythrin. 1.9 is a hamster IgG anti-γδ clonotype mAb used as a negative control.
DISCUSSION
We have generated four mAbs, 8-2D, 8-B3, 4-10D, and 8-B11, specific for the murine αvβ3 vitronectin receptor. Immunoprecipitation analysis, binding competition assays, and platelet staining analysis indicate that 8-B3 and 4-10D are specific for the αv chain and that 8-2D and 8-B11 are specific for the β3 chain. These mAbs were identified based on their ability to block constitutive IL-2 secretion of Vγ1, Vδ6 γδ T cell hybridomas, confirming previous work that demonstrated the involvement of the vitronectin receptor in this spontaneous IL-2 production (15). Studies in which RGD peptides were shown to inhibit such spontaneous IL-2 secretion (15), and transfection experiments in which a Vγ1,Vδ6 TCR was expressed in cells negative for expression of the vitronectin receptor (25), have led to the notion that engagement of the vitronectin receptor is a required coreceptor interaction for this spontaneous response. More recently, it was proposed that engagement of the vitronectin receptor leads to activation directly through the CD3ζ chain and that the Vγ1-Cγ4 chain merely facilitates the interaction between αvβ3 and CD3ζ (26).
The issue of whether the vitronectin receptor functions in Vγ1 cell activation in vivo awaits the development of a system to study responses of primary Vγ1 cells and identification of potential ligands that activate primary Vγ1 cells. However, we were able to detect surface expression of the αvβ3 receptor on Vγ1 transgenic thymocytes and splenocytes (unpublished observations), consistent with a possible function of this receptor on Vγ1 cells in vivo.
Since the mAbs were selected with a functional blocking assay, they were likely to block the binding interactions between αvβ3 and its ligands. We have shown that 8-B3, 4-10D, 8-2D, and 8-B11 can each effectively block cell adhesion to vitronectin, fibronectin, and fibrinogen. These mAbs should prove useful for delineating the involvement of the αv and β3 chains in various cell adhesion interactions.
We have used the four mAbs to study the expression of the αv and β3 chains on lymphocytes in vivo. Previous studies suggested that αvβ3 is not expressed on thymocytes and splenocytes and that expression is only detectable following cell activation or long-term in vitro culture (13, 16). In contrast to these findings, we observed expression of both αv and β3 on certain populations of thymocytes and splenic B and T cells. We also observed expression of αv and β3 on certain populations of bone marrow cells, consistent with previous reports (13, 16). Staining intensities of most of the positive cell populations, and particularly of B cells and pre-B cells, were higher for the anti-αv mAbs than for the anti-β3 mAbs, raising the possibility that on some lymphocyte populations, receptors composed of the αv chain complexed with β chains other than β3 may be expressed. Such receptors as αvβ1 and αvβ5 have been previously described (27, 28).
Stainings of some of the lymphocyte populations with the anti-αvβ3 mAbs, and particularly of pre-B and B cells with the anti-β3 mAbs were of low intensity, however we believe that they represent surface expression of αv and β3 on these cells in vivo, because we observed similar staining patterns with two independent anti-αv and 3 independent anti-β3 mAbs. We cannot formally exclude the possibilities that the stainings represent low-level cross reactivity with different antigens or nonspecific sticking of each of the mAbs to certain cell populations, however we find these interpretations unlikely. Therefore, it is likely that αvβ3 is expressed on resting CD3+ T cells, B cells, and pre-B cells in vivo.
There are two possible explanations for the difference between these results and the previous studies. First, it is possible that the mAbs we selected have higher affinity for αvβ3 than those used previously. Second, we used biotinylated mAbs followed by addition of streptavidin-phycoerythrin to detect expression, which is generally more sensitive than direct detection with FITC-coupled mAbs, as was used in the previous reports. In support of these explanations, we were also unable to detect expression on primary lymphocytes with FITC-coupled mAbs, and we did observe higher expression of αv and β3 on activated lymphocytes relative to primary resting cells (unpublished observations).
Previous reports have described developmentally regulated expression of the β4 integrin and fibronectin receptors on mouse thymocytes (29, 30). Interestingly, we found that expression of αvβ3 also appears to be modulated during T cell development. Thus, we observed high expression of αvβ3 on CD4−CD8− thymocytes, while CD4+CD8+ thymocytes displayed considerably lower αvβ3 expression, and mature CD3+ thymocytes and peripheral T cells were positive for αvβ3 expression. The differential expression of αvβ3 on discrete populations of thymocytes raises the possibility that this receptor is functionally involved in T cell development. The mAbs described here should prove useful in further studying this issue.
Acknowledgments
We thank Linda Graziadei for critical reading of the manuscript, and Pam Woronoff and Emily Rossie for excellent secretarial help. This work was supported by Yakult Honsha Co., Ltd., by National Institutes of Health Grants R35 CA53874 and R37 AI17879 (S.T.), and by Association pour la Recherche sur le Cancer Grant 6969 to P.P. During part of this work, D.J.G. was supported by a National Science Foundation Graduate Research Fellowship.
Footnotes
Abbreviations: ECM, extracellular matrix; RGD, arginine-glycine-aspartic acid; IL-2, interleukin 2; FITC, fluorescein isothiocyanate.
References
- 1.Hynes R O. Cell. 1987;48:549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- 2.Ruoslahti E, Pierschbacher M D. Science. 1987;238:491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 3.Hynes R O. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 4.Hemler M E. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- 5.Springer T A. Nature (London) 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 6.Ruoslahti E, Pierschbacher M D. Cell. 1986;44:517–518. doi: 10.1016/0092-8674(86)90259-x. [DOI] [PubMed] [Google Scholar]
- 7.Pytela R, Pierschbacher M D, Ruoslahti E. Proc Natl Acad Sci USA. 1985;82:5766–5770. doi: 10.1073/pnas.82.17.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki S, Argraves W S, Pytela R, Arai H, Krusius T, Pierschbacher M D, Ruoslahti E. Proc Natl Acad Sci USA. 1986;83:8614–8618. doi: 10.1073/pnas.83.22.8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charo I F, Nannizzi L, Smith J W, Cheresh D A. J Cell Biol. 1990;111:2795–2800. doi: 10.1083/jcb.111.6.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheresh D A, Spiro R C. J Biol Chem. 1987;262:17703–17711. [PubMed] [Google Scholar]
- 11.Charo I F, Fitzgerald L A, Steiner B, Rall S C, Bekeart L S, Phillips D R. Proc Natl Acad Sci USA. 1986;83:8351–8355. doi: 10.1073/pnas.83.21.8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawler J, Weinstein R, Hynes R O. J Cell Biol. 1988;107:2351–2361. doi: 10.1083/jcb.107.6.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maxfield S R, Moulder K, Koning F, Elbe A, Stingl G, Colligan J E, Schevach E M, Yokoyama W M. J Exp Med. 1989;169:2173–2190. doi: 10.1084/jem.169.6.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moulder K, Roberts K, Shevach E M, Colligan J E. J Exp Med. 1991;173:343–347. doi: 10.1084/jem.173.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts K, Yokoyama W M, Kehn P J, Shevach E M. J Exp Med. 1991;173:231–240. doi: 10.1084/jem.173.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi K, Nakamura T, Koyanagi M, Kato K, Hashimoto Y, Yagita H, Okamura K. J Immunol. 1990;145:4371–4379. [PubMed] [Google Scholar]
- 17.Mombaerts P, Iacomini J, Johnson R S, Herrup K, Tonegawa S, Papaioannou V E. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 18.Marcantonio E E, Hynes R O. J Cell Biol. 1988;106:1765–1772. doi: 10.1083/jcb.106.5.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pereira P, Gerber D, Huang S Y, Tonegawa S. J Exp Med. 1995;182:1921–1930. doi: 10.1084/jem.182.6.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J T, Rayburn H, Hynes R O. Development (Cambridge, UK) 1993;119:1093–1105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
- 21.Hynes R O. Proc Natl Acad Sci USA. 1973;70:3170–3174. doi: 10.1073/pnas.70.11.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horton M. Int J Exp Pathol. 1990;71:741–759. [PMC free article] [PubMed] [Google Scholar]
- 23.Isenberg W M, McEver R P, Phillips D R, Shuman M A, Bainton D F. J Cell Biol. 1987;104:1655–1663. doi: 10.1083/jcb.104.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lam S C, Plow E F, D’Souza S E, Cheresh D A, Frelinger A L, III, Ginsberg M H. J Biol Chem. 1989;264:3742–3749. [PubMed] [Google Scholar]
- 25.Kikuchi G E, Roberts K, Shevach E M, Colligan J E. J Immunol. 1992;148:1302–1307. [PubMed] [Google Scholar]
- 26.Sturmhofel K, Brando C, Martinon F, Shevach E M, Colligan J E. J Immunol. 1995;154:2104–2111. [PubMed] [Google Scholar]
- 27.Vogel B E, Tarone G, Giancotti F G, Gailit J, Ruoslahti E. J Biol Chem. 1990;265:5934–5937. [PubMed] [Google Scholar]
- 28.Smith J W, Vestal D J, Irwin S V, Burke T A, Cheresh D A. J Biol Chem. 1990;265:11008–11013. [PubMed] [Google Scholar]
- 29.Wadsworth S, Halvorson M J, Colligan J E. J Immunol. 1992;149:421–428. [PubMed] [Google Scholar]
- 30.Cardarelli P M, Crispe I N, Pierschbacher M D. J Cell Biol. 1988;106:2183–2190. doi: 10.1083/jcb.106.6.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]


