Abstract
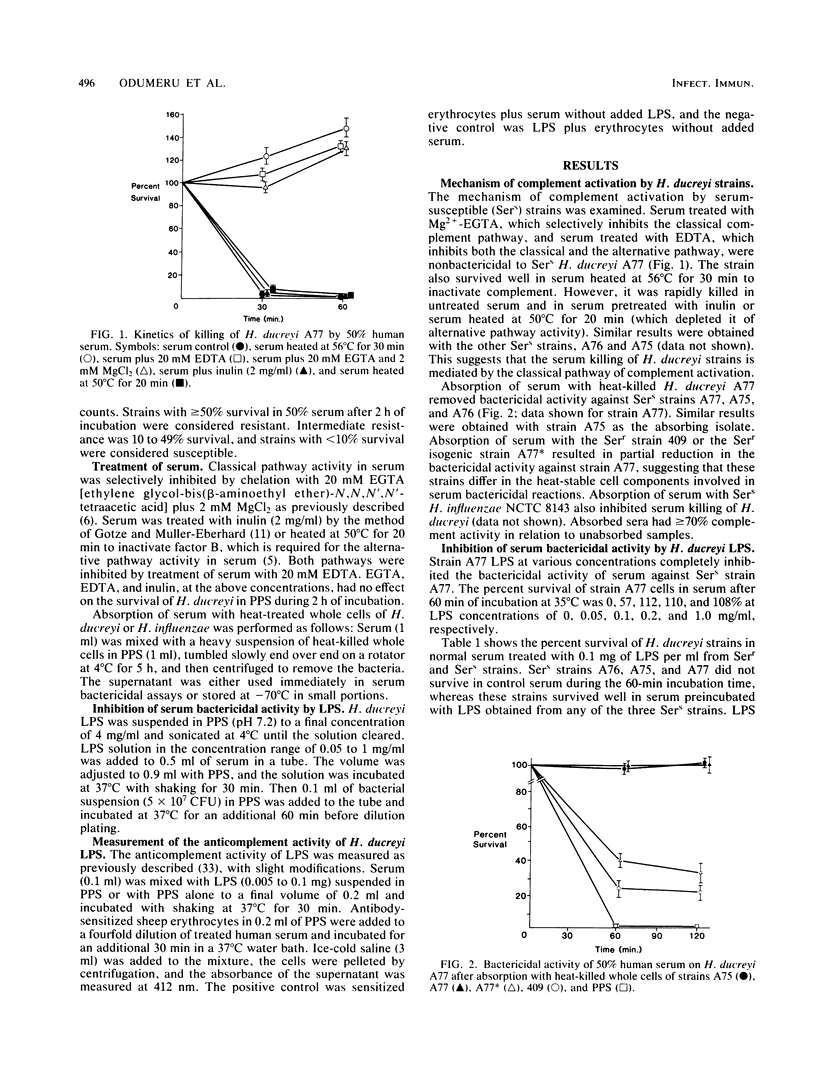
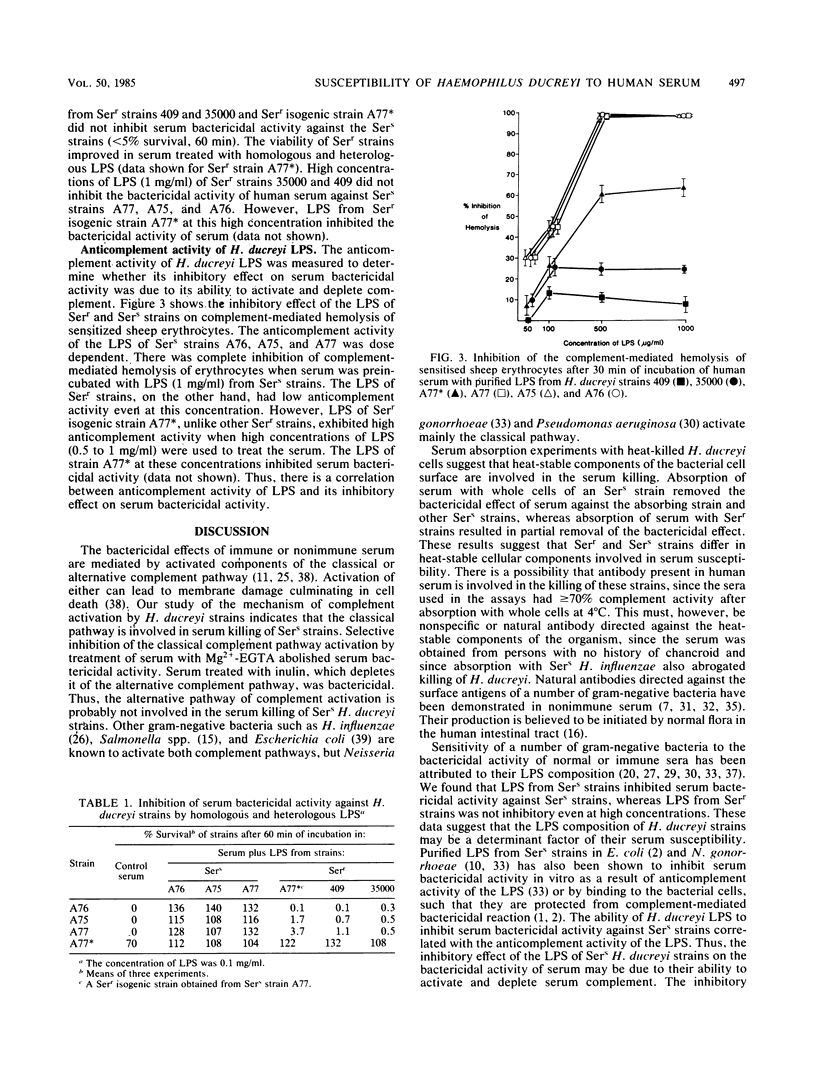
The role of lipopolysaccharide (LPS) in the susceptibility of Haemophilus ducreyi to human serum and the mechanism of complement activation by serum-susceptible (Sers) strains were investigated. Serum treated with 2 mM Mg2+ and 20 mM ethylene glycol-bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid was nonbactericidal, but inulin-treated serum remained bactericidal. Absorption of serum with heat-killed whole cells of an Sers strain removed its bactericidal activity against the absorbing strain and also against other Sers strains. LPS obtained from Sers strains inhibited the bactericidal activity of serum against all Sers strains, whereas LPS from serum-resistant (Serr) strains and an Serr isogenic strain did not. However, high concentrations of LPS from the Serr strain inhibited the bactericidal activity of serum, an indication that part of the structural site involved in serum susceptibility is retained in the LPS of this strain. The LPS of Sers strains exhibited higher anticomplement activity than the LPS of Serr strains. These findings suggest that the classical pathway of complement activation is involved in the serum killing of H. ducreyi and that LPS composition may contribute to their susceptibility to complement-mediated serum bactericidal activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. J., Scott G. K. Comparison of the effects of different lipopolysaccharides on the serum bactericidal reactions of two strains of Escherichia coli. Infect Immun. 1981 Feb;31(2):831–832. doi: 10.1128/iai.31.2.831-832.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R. J., Scott G. K. The effect of purified lipopolysaccharide on the bactericidal reaction of human serum complement. J Gen Microbiol. 1980 Mar;117(1):65–72. doi: 10.1099/00221287-117-1-65. [DOI] [PubMed] [Google Scholar]
- Corrall C. J., Winkelstein J. A., Moxon E. R. Participation of complement in host defense against encapsulated Haemophilus influenzae types a, c, and d. Infect Immun. 1982 Mar;35(3):759–763. doi: 10.1128/iai.35.3.759-763.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidinger D., Bello E., Mates A. The heterocytotoxicity of human serum. I. Activation of the alternative complement pathway by heterologous target cells. Cell Immunol. 1977 Mar 1;29(1):174–186. doi: 10.1016/0008-8749(77)90286-6. [DOI] [PubMed] [Google Scholar]
- Fine D. P., Marney S. R., Jr, Colley D. G., Sergent J. S., Des Prez R. M. C3 shunt activation in human serum chelated with EGTA. J Immunol. 1972 Oct;109(4):807–809. [PubMed] [Google Scholar]
- GAINES S., LANDY M. Prevalence of antibody to Pseudomonas in normal human sera. J Bacteriol. 1955 Jun;69(6):628–633. doi: 10.1128/jb.69.6.628-633.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O. The role of the physical state of lipopolysaccharides in the interaction with complement. High molecular weight as prerequisite for the expression of anti-complementary activity. Eur J Biochem. 1976 Jun 1;65(2):403–408. doi: 10.1111/j.1432-1033.1976.tb10354.x. [DOI] [PubMed] [Google Scholar]
- Glynn A. A., Howard C. J. The sensitivity to complement of strains of Escherichia coli related to their K antigens. Immunology. 1970 Mar;18(3):331–346. [PMC free article] [PubMed] [Google Scholar]
- Glynn A. A., Ward M. E. Nature and Heterogeneity of the Antigens of Neisseria gonorrhoeae Involved in the Serum Bactericidal Reaction. Infect Immun. 1970 Aug;2(2):162–168. doi: 10.1128/iai.2.2.162-168.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt J. F., Mayer L. W., Wang S. P., Buchanan T. M. Neisseria gonorrhoeae acquire a new principal outer-membrane protein when transformed to resistance to serum bactericidal activity. Infect Immun. 1978 Apr;20(1):267–272. doi: 10.1128/iai.20.1.267-272.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Hammer C. H., Brown E. J., Cole R. J., Frank M. M. Studies on the mechanism of bacterial resistance to complement-mediated killing. I. Terminal complement components are deposited and released from Salmonella minnesota S218 without causing bacterial death. J Exp Med. 1982 Mar 1;155(3):797–808. doi: 10.1084/jem.155.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe W. R., Kaijser B., Olling S., Uwaydah M., Hanson L. A. Escherichia coli in bacteremia: K and O antigens and serum sensitivity of strains from adults and neonates. J Infect Dis. 1978 Jul;138(1):33–41. doi: 10.1093/infdis/138.1.33. [DOI] [PubMed] [Google Scholar]
- Moll A., Manning P. A., Timmis K. N. Plasmid-determined resistance to serum bactericidal activity: a major outer membrane protein, the traT gene product, is responsible for plasmid-specified serum resistance in Escherichia coli. Infect Immun. 1980 May;28(2):359–367. doi: 10.1128/iai.28.2.359-367.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Kline L. F. Activation of the classical and properdin pathways of complement by bacterial lipopolysaccharides (LPS). J Immunol. 1977 Jan;118(1):362–368. [PubMed] [Google Scholar]
- Munn C. B., Ishiguro E. E., Kay W. W., Trust T. J. Role of surface components in serum resistance of virulent Aeromonas salmonicida. Infect Immun. 1982 Jun;36(3):1069–1075. doi: 10.1128/iai.36.3.1069-1075.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschel L. H., Larsen L. J. The sensitivity of smooth and rough gram-negative bacteria to the immune bactericidal reaction. Proc Soc Exp Biol Med. 1970 Jan;133(1):345–348. doi: 10.3181/00379727-133-34472. [DOI] [PubMed] [Google Scholar]
- Nelson B. W., Roantree R. J. Analyses of lipopolysaccharides extracted from penicillin-resistant, serum-sensitive salmonella mutants. J Gen Microbiol. 1967 Aug;48(2):179–188. doi: 10.1099/00221287-48-2-179. [DOI] [PubMed] [Google Scholar]
- Odumeru J. A., Wiseman G. M., Ronald A. R. Virulence factors of Haemophilus ducreyi. Infect Immun. 1984 Feb;43(2):607–611. doi: 10.1128/iai.43.2.607-611.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olling S. Sensitivity of gram-negative bacilli to the serum bactericidal activity: a marker of the host-parasite relationship in acute and persisting infections. Scand J Infect Dis Suppl. 1977;(10):1–40. doi: 10.3109/inf.1977.9.suppl-10.01. [DOI] [PubMed] [Google Scholar]
- Pangburn M. K. Activation of complement via the alternative pathway. Fed Proc. 1983 Jan;42(1):139–143. [PubMed] [Google Scholar]
- Quinn P. H., Crosson F. J., Jr, Winkelstein J. A., Moxon E. R. Activation of the alternative complement pathway by Haemophilus influenzae type B. Infect Immun. 1977 Apr;16(1):400–402. doi: 10.1128/iai.16.1.400-402.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice P. A., Kasper D. L. Characterization of gonococcal antigens responsible for induction of bactericidal antibody in disseminated infection. J Clin Invest. 1977 Nov;60(5):1149–1158. doi: 10.1172/JCI108867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roantree R. J., Rantz L. A. A STUDY OF THE RELATIONSHIP OF THE NORMAL BACTERICIDAL ACTIVITY OF HUMAN SERUM TO BACTERIAL INFECTION. J Clin Invest. 1960 Jan;39(1):72–81. doi: 10.1172/JCI104029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley D. Sensitivity of rough gram-negative bacteria to the bactericidal action of serum. J Bacteriol. 1968 May;95(5):1647–1650. doi: 10.1128/jb.95.5.1647-1650.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller N. L., Alazard M. J., Borowski R. S. Serum sensitivity of a Pseudomonas aeruginosa mucoid strain. Infect Immun. 1984 Sep;45(3):748–755. doi: 10.1128/iai.45.3.748-755.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoolnik G. K., Buchanan T. M., Holmes K. K. Gonococci causing disseminated gonococcal infection are resistant to the bactericidal action of normal human sera. J Clin Invest. 1976 Nov;58(5):1163–1173. doi: 10.1172/JCI108569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab G. E., Reeves P. R. Comparison of the bactericidal activity of different vertebrate sera. J Bacteriol. 1966 Jan;91(1):106–112. doi: 10.1128/jb.91.1.106-112.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Joiner K., Guymon L. F., Cohen M. S., Sparling P. F. Serum sensitivity of Neisseria gonorrhoeae: the role of lipopolysaccharide. J Infect Dis. 1984 Feb;149(2):175–183. doi: 10.1093/infdis/149.2.175. [DOI] [PubMed] [Google Scholar]
- Simberkoff M. S., Ricupero I., Rahal J. J., Jr Host resistance to Serratia marcescens infection: serum bactericidal activity and phagocytosis by normal blood leukocytes. J Lab Clin Med. 1976 Feb;87(2):206–217. [PubMed] [Google Scholar]
- Skarnes R. C. Humoral bactericidal systems: nonspecific and specific mechanisms. Infect Immun. 1978 Feb;19(2):515–522. doi: 10.1128/iai.19.2.515-522.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton A., Schneerson R., Kendall-Morris S., Robbins J. B. Differential complement resistance mediates virulence of Haemophilus influenzae type b. Infect Immun. 1982 Jan;35(1):95–104. doi: 10.1128/iai.35.1.95-104.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W. Bactericidal and bacteriolytic activity of serum against gram-negative bacteria. Microbiol Rev. 1983 Mar;47(1):46–83. doi: 10.1128/mr.47.1.46-83.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W. Genetical studies of serum resistance in Escherichia coli. J Gen Microbiol. 1975 Jul;89(1):57–66. doi: 10.1099/00221287-89-1-57. [DOI] [PubMed] [Google Scholar]
- Taylor P. W., Parton R. A protein factor associated with serum resistance in Escherichia coli. J Med Microbiol. 1977 May;10(2):225–232. doi: 10.1099/00222615-10-2-225. [DOI] [PubMed] [Google Scholar]
- Vosti K. L., Randall E. Sensitivity of serologically classified strains of escherichia coli of human origin to the serum bactericidal system. Am J Med Sci. 1970 Feb;259(2):114–119. doi: 10.1097/00000441-197002000-00005. [DOI] [PubMed] [Google Scholar]



