Abstract
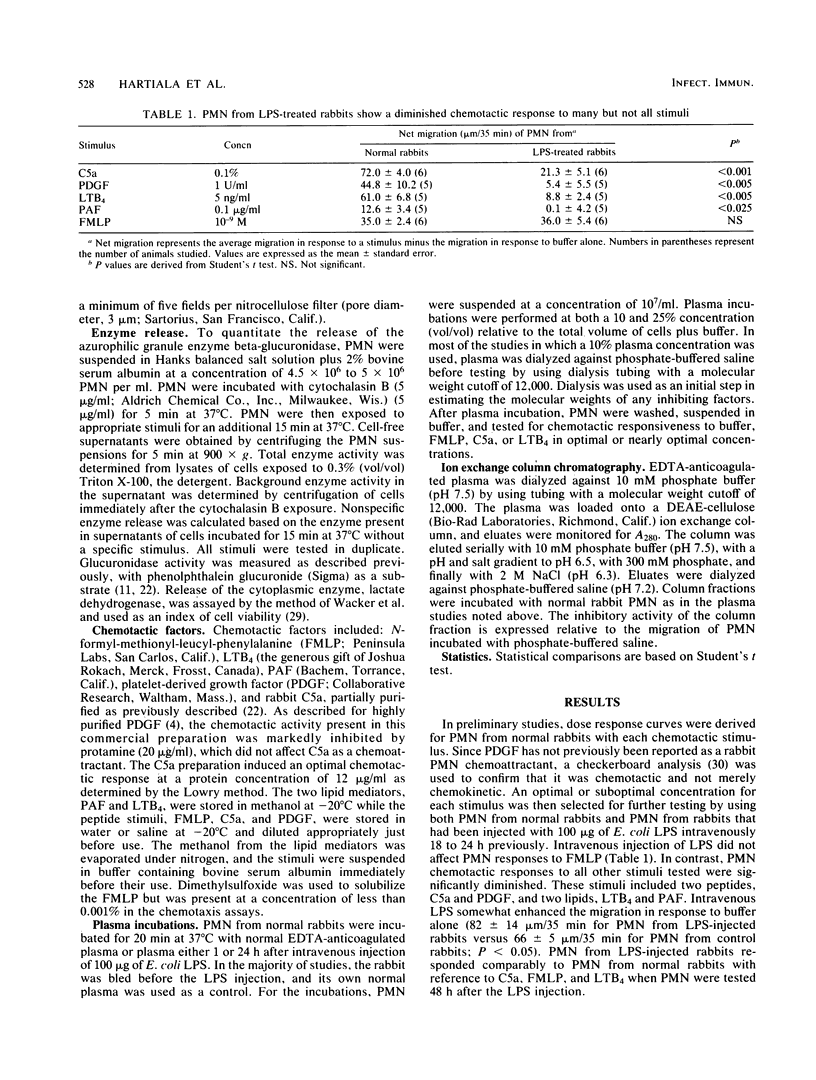
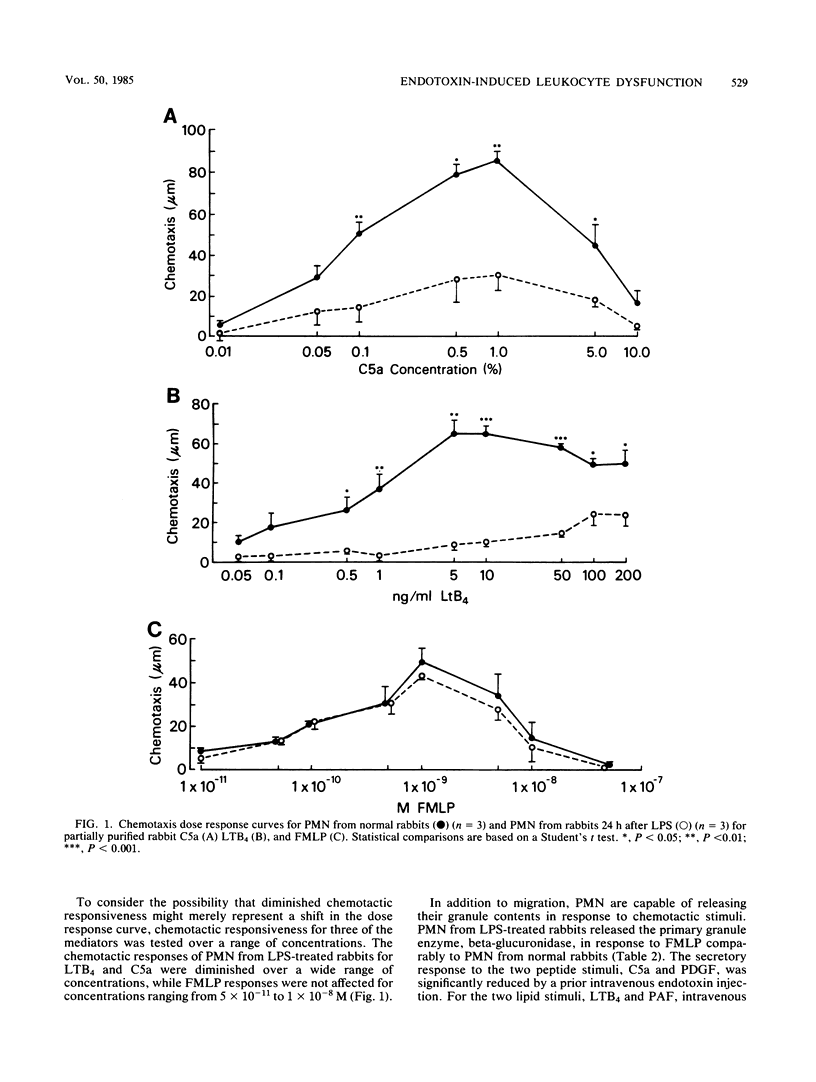
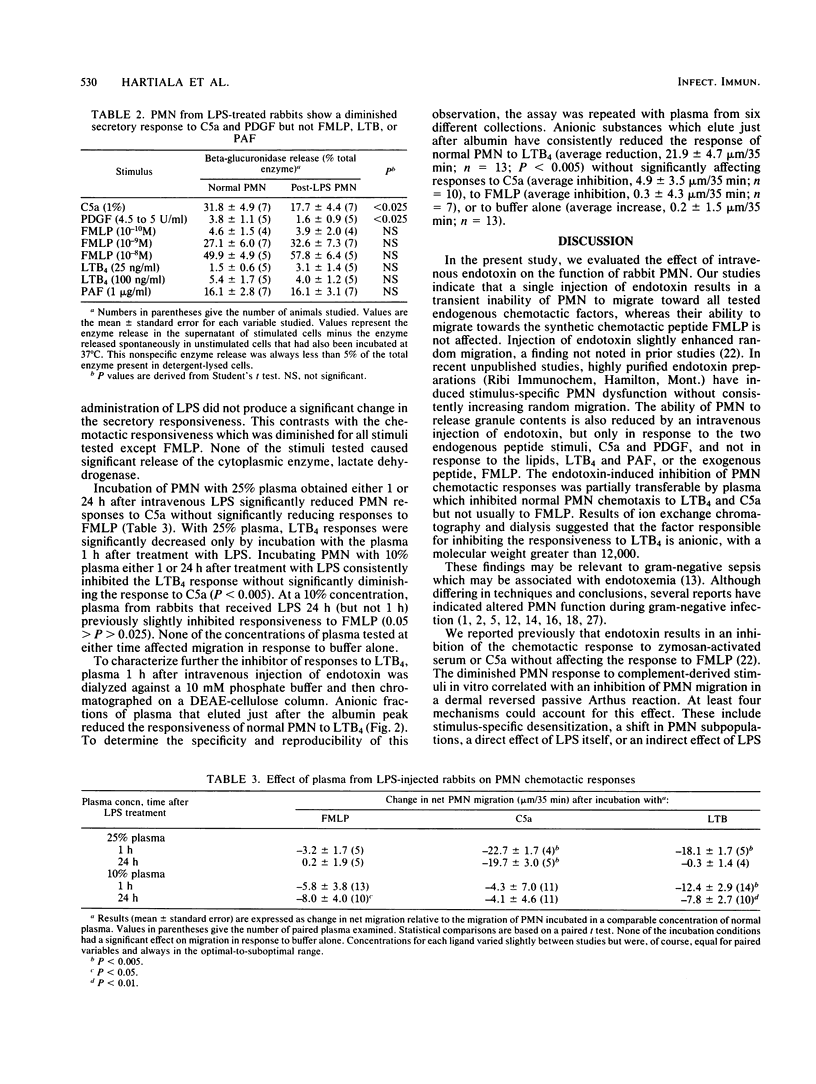
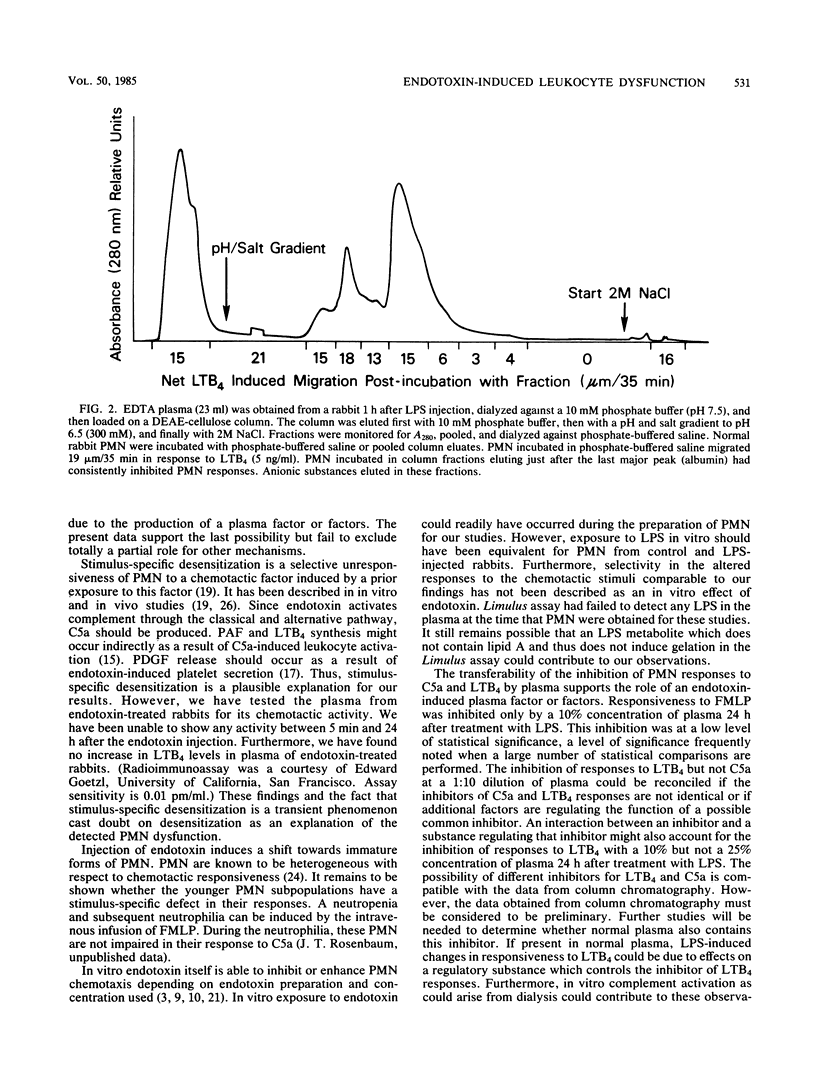
To assess the mechanism and specificity of polymorphonuclear leukocyte (PMN) dysfunction induced by endotoxin, rabbits were injected intravenously with 100 micrograms of Escherichia coli endotoxin, and PMN function was studied 18 to 24 h later. Compared to PMN from normal rabbits, peripheral blood PMN from rabbits injected with endotoxin showed diminished chemotactic responsiveness to two endogenous peptides, C5a (complement) and platelet-derived growth factor, and to two endogenous lipids, leukotriene B4 and platelet-activating factor. The chemotactic response to the synthetic chemotactic peptide, N-formyl-methionyl-leucyl-phenylalanine (FMLP), was unimpaired. In contrast to migration, endotoxin injection resulted in inhibition of the secretory response to the two endogenous peptides but not to the lipids or to FMLP. At a 1:4 (vol/vol) dilution, the plasma either 1 or 24 h after the endotoxin injection inhibited normal PMN chemotactic responses to C5a but not to FMLP. Similarly, at a 1:10 dilution, this plasma inhibited normal PMN chemotactic responses to leukotriene B4. The factor responsible for inhibiting responses to leukotriene B4 was anionic, specific for leukotriene B4 responses, and greater than 12,000 daltons. These data may be relevant to understanding PMN dysfunction during gram-negative sepsis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Althaus D., Keller H. U., Hess M. W., Cottier H. Impaired neutrophil locomotion during acute bacterial infections. Int Arch Allergy Appl Immunol. 1980;61(3):321–328. doi: 10.1159/000232454. [DOI] [PubMed] [Google Scholar]
- Christou N. V., Meakins J. L. Neutrophil function in surgical patients: two inhibitors of granulocyte chemotaxis associated with sepsis. J Surg Res. 1979 Apr;26(4):355–364. doi: 10.1016/0022-4804(79)90020-9. [DOI] [PubMed] [Google Scholar]
- Dahinden C., Galanos C., Fehr J. Granulocyte activation by endotoxin. I. Correlation between adherence and other granulocyte functions, and role of endotoxin structure on biologic activity. J Immunol. 1983 Feb;130(2):857–862. [PubMed] [Google Scholar]
- Deuel T. F., Senior R. M., Huang J. S., Griffin G. L. Chemotaxis of monocytes and neutrophils to platelet-derived growth factor. J Clin Invest. 1982 Apr;69(4):1046–1049. doi: 10.1172/JCI110509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei P. C., Hermanovicz A., Pécoud A. Chemotaxis of human polymorphonuclears in vitro. V. Role of the nonsegmented neutrophils and of the experimental conditions in the impairment of chemotaxis observed during bacterial infections. J Lab Clin Med. 1978 Oct;92(4):577–584. [PubMed] [Google Scholar]
- Goetzl E. J., Pickett W. C. The human PMN leukocyte chemotactic activity of complex hydroxy-eicosatetraenoic acids (HETEs). J Immunol. 1980 Oct;125(4):1789–1791. [PubMed] [Google Scholar]
- Goldman D. W., Goetzl E. J. Heterogeneity of human polymorphonuclear leukocyte receptors for leukotriene B4. Identification of a subset of high affinity receptors that transduce the chemotactic response. J Exp Med. 1984 Apr 1;159(4):1027–1041. doi: 10.1084/jem.159.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie L. A., McPhail L. C., Henson P. M., Johnston R. B., Jr Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. J Exp Med. 1984 Dec 1;160(6):1656–1671. doi: 10.1084/jem.160.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henricks P. A., van der Tol M. E., Thyssen R. M., van Asbeck B. S., Verhoef J. Escherichia coli lipopolysaccharides diminish and enhance cell function of human polymorphonuclear leukocytes. Infect Immun. 1983 Jul;41(1):294–301. doi: 10.1128/iai.41.1.294-301.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M. The immunologic release of constituents from neutrophil leukocytes. II. Mechanisms of release during phagocytosis, and adherence to nonphagocytosable surfaces. J Immunol. 1971 Dec;107(6):1547–1557. [PubMed] [Google Scholar]
- Hill H. R., Gerrard J. M., Hogan N. A., Quie P. G. Hyperactivity of neutrophil leukotactic responses during active bacterial infection. J Clin Invest. 1974 Apr;53(4):996–1002. doi: 10.1172/JCI107666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J., Poore T. E., Zauber N. P., Oser R. S. Detection of endotoxin in the blood of patients with sepsis due to gran-negative bacteria. N Engl J Med. 1970 Dec 10;283(24):1313–1316. doi: 10.1056/NEJM197012102832404. [DOI] [PubMed] [Google Scholar]
- Link A. S., Jr, Bass D. A., McCall C. E. Altered neutrophil migration during bacterial infection associated with a serum modulator of cellular motility. J Infect Dis. 1979 Oct;140(4):517–526. doi: 10.1093/infdis/140.4.517. [DOI] [PubMed] [Google Scholar]
- Lynch J. M., Lotner G. Z., Betz S. J., Henson P. M. The release of a platelet-activating factor by stimulated rabbit neutrophils. J Immunol. 1979 Sep;123(3):1219–1226. [PubMed] [Google Scholar]
- McCall C. E., Caves J., Cooper R., DeChatlet L. Functional characteristics of human toxic neutrophils. J Infect Dis. 1971 Jul;124(1):68–75. doi: 10.1093/infdis/124.1.68. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Kline L. F., Oades Z. G., Henson P. M. Mechanisms of lipopolysaccharide-initiated rabbit platelet responses: alternative complement pathway dependence of the lytic response. Infect Immun. 1978 Jun;20(3):744–751. doi: 10.1128/iai.20.3.744-751.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat A. G., Baum J. Polymorphonuclear leucocyte chemotaxis in patients with bacterial infections. Br Med J. 1971 Sep 11;3(5775):617–619. doi: 10.1136/bmj.3.5775.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty J. T., Kreutzer D. L., Showell H. J., Vitkauskas G., Becker E. L., Ward P. A. Selective neutrophil desensitization to chemotactic factors. J Cell Biol. 1979 Mar;80(3):564–572. doi: 10.1083/jcb.80.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty J. T., Lees C. J., Miller C. H., McCall C. E., Lewis J. C., Love S. H., Wykle R. L. Selective desensitization of neutrophils: further studies with 1-O-alkyl-sn-glycero-3-phosphocholine analogues. J Immunol. 1981 Aug;127(2):731–737. [PubMed] [Google Scholar]
- Proctor R. A. Endotoxin in vitro interactions with human neutrophils: depression of chemiluminescence, oxygen consumption, superoxide production, and killing. Infect Immun. 1979 Sep;25(3):912–921. doi: 10.1128/iai.25.3.912-921.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum J. T., Hartiala K. T., Webster R. O., Howes E. L., Jr, Goldstein I. M. Antiinflammatory effects of endotoxin. Inhibition of rabbit polymorphonuclear leukocyte responses to complement (C5)-derived peptides in vivo and in vitro. Am J Pathol. 1983 Dec;113(3):291–299. [PMC free article] [PubMed] [Google Scholar]
- Ruddy S., Austen K. F., Goetzl E. J. Chemotactic activity derived from interaction of factors D and B of the properdin pathway with cobra venom factor or C3B. J Clin Invest. 1975 Mar;55(3):587–592. doi: 10.1172/JCI107966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligmann B., Chused T. M., Gallin J. I. Differential binding of chemoattractant peptide to subpopulations of human neutrophils. J Immunol. 1984 Nov;133(5):2641–2646. [PubMed] [Google Scholar]
- Serhan C. N., Radin A., Smolen J. E., Korchak H., Samuelsson B., Weissmann G. Leukotriene B4 is a complete secretagogue in human neutrophils: a kinetic analysis. Biochem Biophys Res Commun. 1982 Aug;107(3):1006–1012. doi: 10.1016/0006-291x(82)90622-2. [DOI] [PubMed] [Google Scholar]
- Skubitz K. M., Craddock P. R. Reversal of hemodialysis granulocytopenia and pulmonary leukostasis: A clinical manifestation of selective down-regulation of granulocyte responses to C5adesarg. J Clin Invest. 1981 May;67(5):1383–1391. doi: 10.1172/JCI110166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomkin J. S., Jenkins M. K., Nelson R. D., Chenoweth D., Simmons R. L. Neutrophil dysfunction in sepsis. II. Evidence for the role of complement activation products in cellular deactivation. Surgery. 1981 Aug;90(2):319–327. [PubMed] [Google Scholar]
- ULMER D. D., VALLEE B. L., WACKER W. E. Metalloenzymes and myocardial infarction. II. Malic and lactic dehydrogenase activities and zinc concentrations in serum. N Engl J Med. 1956 Sep 6;255(10):450–456. doi: 10.1056/NEJM195609062551001. [DOI] [PubMed] [Google Scholar]
- Verghese M. W., Snyderman R. Differential anti-inflammatory effects of LPS in susceptible and resistant mouse strains. J Immunol. 1981 Jul;127(1):288–293. [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]


