Abstract
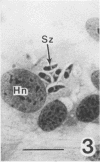
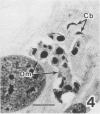
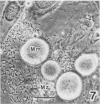
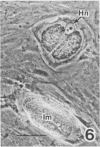
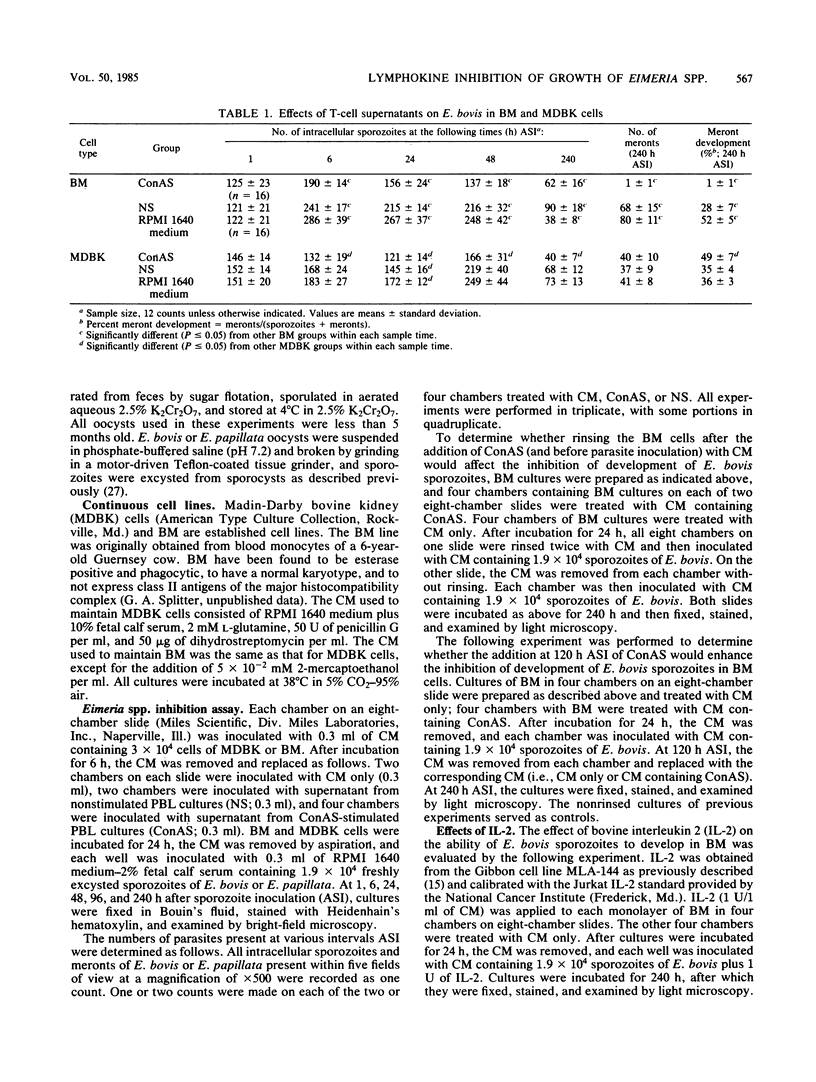
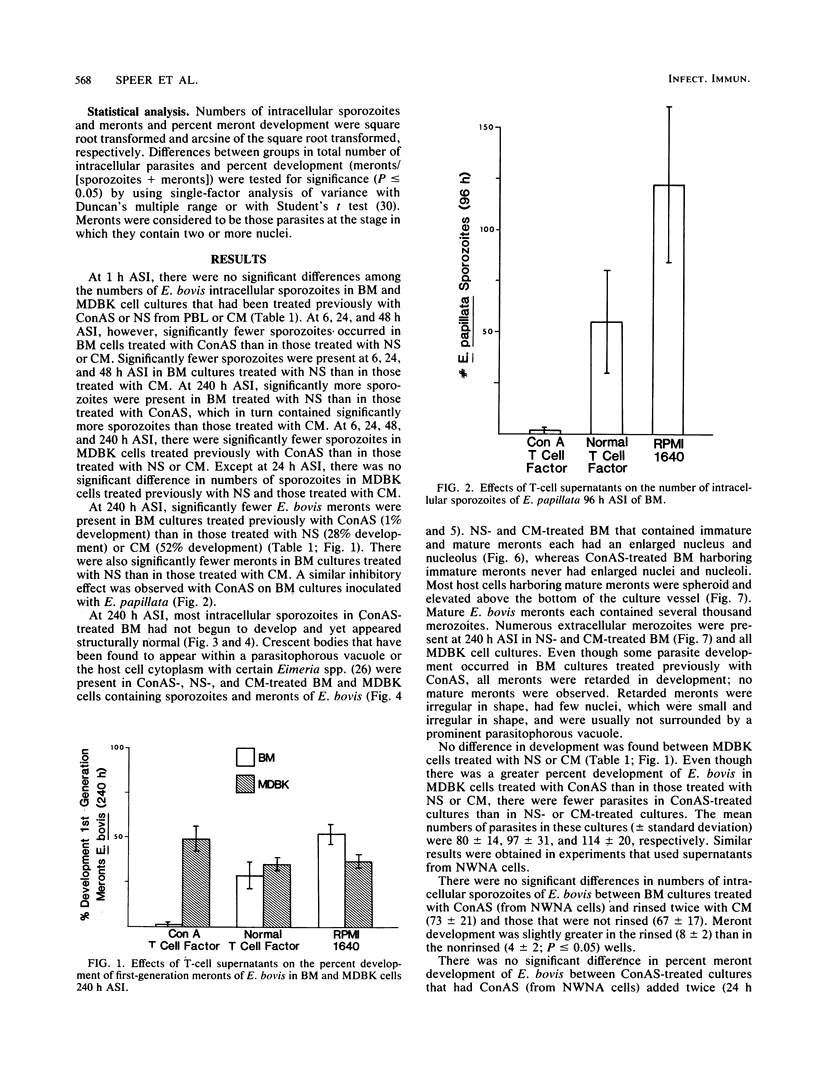
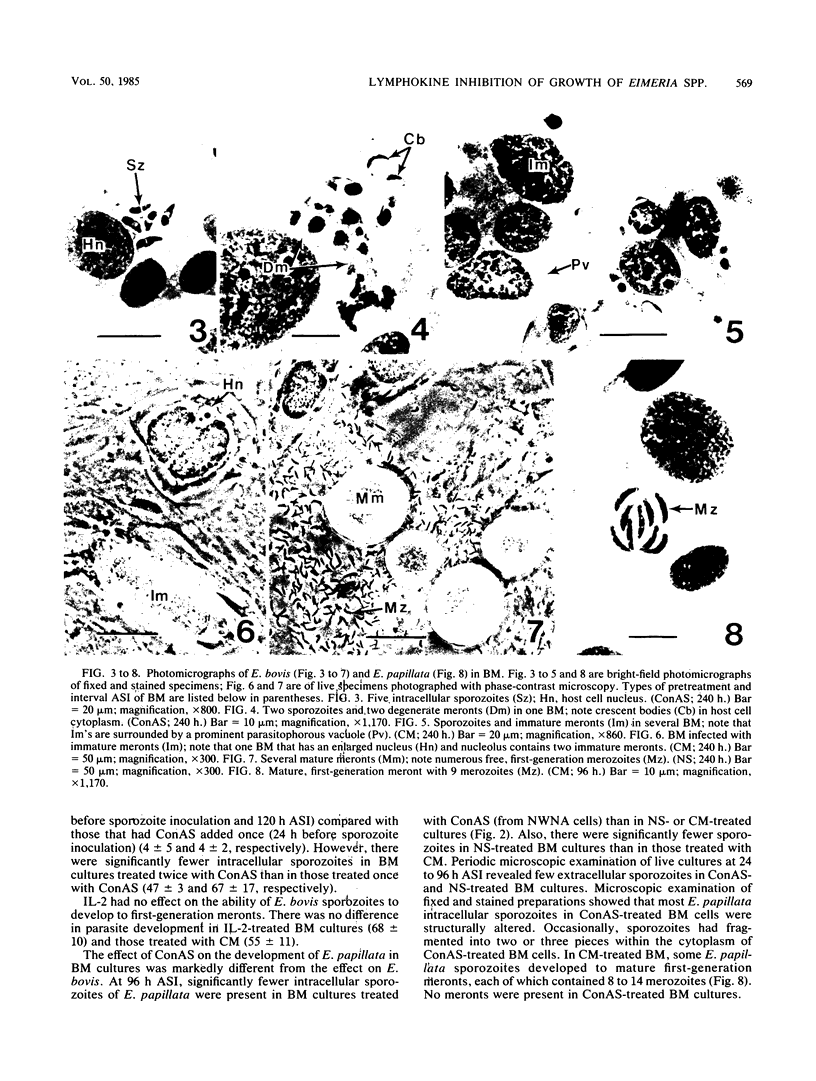
Sporozoites of Eimeria bovis penetrated and developed normally to first-generation meronts in bovine monocytes (BM) and Madin-Darby bovine kidney (MDBK) cells that had been pretreated with culture medium (CM) or supernatant (NS) from nonstimulated bovine T cells. At 240 h after sporozoite inoculation (ASI), the mean percent development (meronts/[sporozoites + meronts]) in CM- and NS-pretreated BM was 52 and 28%, respectively; values for MDBK cells were 36 and 35%, respectively. Pretreatment of BM and MDBK cells with supernatant (ConAS) from concanavalin A-stimulated bovine T cells had no effect on the ability of sporozoites to penetrate cells; however, at 240 h ASI, only 1% of the sporozoites in ConAS-pretreated BM cultures had developed to meronts. In contrast, ConAS had no adverse effect on the ability of E. bovis sporozoites to develop to first-generation meronts in MDBK cells. At 240 h ASI, E. bovis meronts in ConAS-pretreated BM were abnormal in appearance and retarded in development, whereas sporozoites appeared structurally normal by light microscopy. Pretreatment of BM with ConAS had no effect on the ability of sporozoites of Eimeria papillata (Apicomplexa) to penetrate cells. Sporozoites of E. papillata did not develop to meronts in ConAS-pretreated BM and, in contrast to E. bovis, most sporozoites were destroyed intracellularly.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. E., Hagemo A., Knoblock K., Dubey J. P. Toxoplasma gondii: microassay to differentiate toxoplasma inhibiting factor and interleukin 2. Exp Parasitol. 1983 Jun;55(3):320–330. doi: 10.1016/0014-4894(83)90029-2. [DOI] [PubMed] [Google Scholar]
- Baker P. E., Knoblock K. F. Bovine costimulator. I. Production kinetics, partial purification, and quantification in serum-free Iscove's medium. Vet Immunol Immunopathol. 1982 Jul;3(4):365–379. doi: 10.1016/0165-2427(82)90020-4. [DOI] [PubMed] [Google Scholar]
- Byrne G. I., Faubion C. L. Lymphokine-mediated microbistatic mechanisms restrict Chlamydia psittaci growth in macrophages. J Immunol. 1982 Jan;128(1):469–474. [PubMed] [Google Scholar]
- Chinchilla M., Frenkel J. K. Mediation of immunity to intracellular infection (Toxoplasma and Besnoitia) within somatic cells. Infect Immun. 1978 Mar;19(3):999–1012. doi: 10.1128/iai.19.3.999-1012.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla M., Frenkel J. K. Specific mediation of cellular immunity to Toxoplasma gondii in somatic cells of mice. Infect Immun. 1984 Dec;46(3):862–866. doi: 10.1128/iai.46.3.862-866.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMMOND D. M., ANDERSEN F. L., MINER M. L. The occurrence of a second asexual generation in the life cycle of Eimeria bovis in calves. J Parasitol. 1963 Jun;49:428–434. [PubMed] [Google Scholar]
- HAMMOND D. M., ANDERSEN F. L., MINER M. L. The site of the immune reaction against Eimeria bovis in calves. J Parasitol. 1963 Jun;49:415–424. [PubMed] [Google Scholar]
- Hoover D. L., Nacy C. A. Macrophage activation to kill Leishmania tropica: defective intracellular killing of amastigotes by macrophages elicited with sterile inflammatory agents. J Immunol. 1984 Mar;132(3):1487–1493. [PubMed] [Google Scholar]
- Krotoski W. A., Krotoski D. M., Garnham P. C., Bray R. S., Killick-Kendrick R., Draper C. C., Targett G. A., Guy M. W. Relapses in primate malaria: discovery of two populations of exoerythrocytic stages. Preliminary note. Br Med J. 1980 Jan 19;280(6208):153–154. doi: 10.1136/bmj.280.6208.153-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt W. C. Some problems of host and parasite interactions in the coccidia. J Protozool. 1976 May;23(2):287–290. doi: 10.1111/j.1550-7408.1976.tb03772.x. [DOI] [PubMed] [Google Scholar]
- Meltzer M. S., Benjamin W. R., Farrar J. J. Macrophage activation for tumor cytotoxicity: induction of macrophage tumoricidal activity by lymphokines from EL-4, a continuous T cell line. J Immunol. 1982 Dec;129(6):2802–2807. [PubMed] [Google Scholar]
- Miller-Edge M., Splitter G. A. Bovine interleukin 2 (IL-2) production and activity on bovine and murine cell lines. Vet Immunol Immunopathol. 1984 Sep;7(2):119–130. doi: 10.1016/0165-2427(84)90013-8. [DOI] [PubMed] [Google Scholar]
- Nacy C. A., Fortier A. H., Pappas M. G., Henry R. R. Susceptibility of inbred mice to Leishmania tropica infection: correlation of susceptibility with in vitro defective macrophage microbicidal activities. Cell Immunol. 1983 Apr 15;77(2):298–307. doi: 10.1016/0008-8749(83)90030-8. [DOI] [PubMed] [Google Scholar]
- Nacy C. A., Meltzer M. S., Leonard E. J., Wyler D. J. Intracellular replication and lymphokine-induced destruction of Leishmania tropica in C3H/HeN mouse macrophages. J Immunol. 1981 Dec;127(6):2381–2386. [PubMed] [Google Scholar]
- Nacy C. A., Meltzer M. S. Macrophages in resistance to rickettsial infection: macrophage activation in vitro for killing of Rickettsia tsutsugamushi. J Immunol. 1979 Dec;123(6):2544–2549. [PubMed] [Google Scholar]
- Nogueira N., Cohn Z. A. Trypanosoma cruzi: in vitro induction of macrophage microbicidal activity. J Exp Med. 1978 Jul 1;148(1):288–300. doi: 10.1084/jem.148.1.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster C. N., Nacy C. A. Macrophage activation to kill Leishmania tropica: kinetics of macrophage response to lymphokines that induce antimicrobial activities against amastigotes. J Immunol. 1984 Mar;132(3):1494–1500. [PubMed] [Google Scholar]
- Pfefferkorn E. R., Guyre P. M. Inhibition of growth of Toxoplasma gondii in cultured fibroblasts by human recombinant gamma interferon. Infect Immun. 1984 May;44(2):211–216. doi: 10.1128/iai.44.2.211-216.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R. D., Pace J. L., Russell S. W., Altman A., Katz D. H. Macrophage-activating factor produced by a T cell hybridoma: physiochemical and biosynthetic resemblance to gamma-interferon. J Immunol. 1983 Aug;131(2):826–832. [PubMed] [Google Scholar]
- Shirahata T., Shimizu K. Growth inhibition of Toxoplasma gondii in cell cultures treated with murine type II interferon. Nihon Juigaku Zasshi. 1982 Dec;44(6):865–871. doi: 10.1292/jvms1939.44.865. [DOI] [PubMed] [Google Scholar]
- Shirahata T., Shimizu K. Production and properties of immune interferon from spleen cell cultures of Toxoplasma-infected mice. Microbiol Immunol. 1980;24(11):1109–1120. doi: 10.1111/j.1348-0421.1980.tb02915.x. [DOI] [PubMed] [Google Scholar]
- Sibert G. J., Speer C. A. Fine structure of nuclear division and microgametogony of Eimeria nieschulzi Dieben, 1924. Z Parasitenkd. 1981;66(2):179–189. doi: 10.1007/BF00925725. [DOI] [PubMed] [Google Scholar]
- Turco J., Thompson H. A., Winkler H. H. Interferon-gamma inhibits growth of Coxiella burnetii in mouse fibroblasts. Infect Immun. 1984 Sep;45(3):781–783. doi: 10.1128/iai.45.3.781-783.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]