Abstract
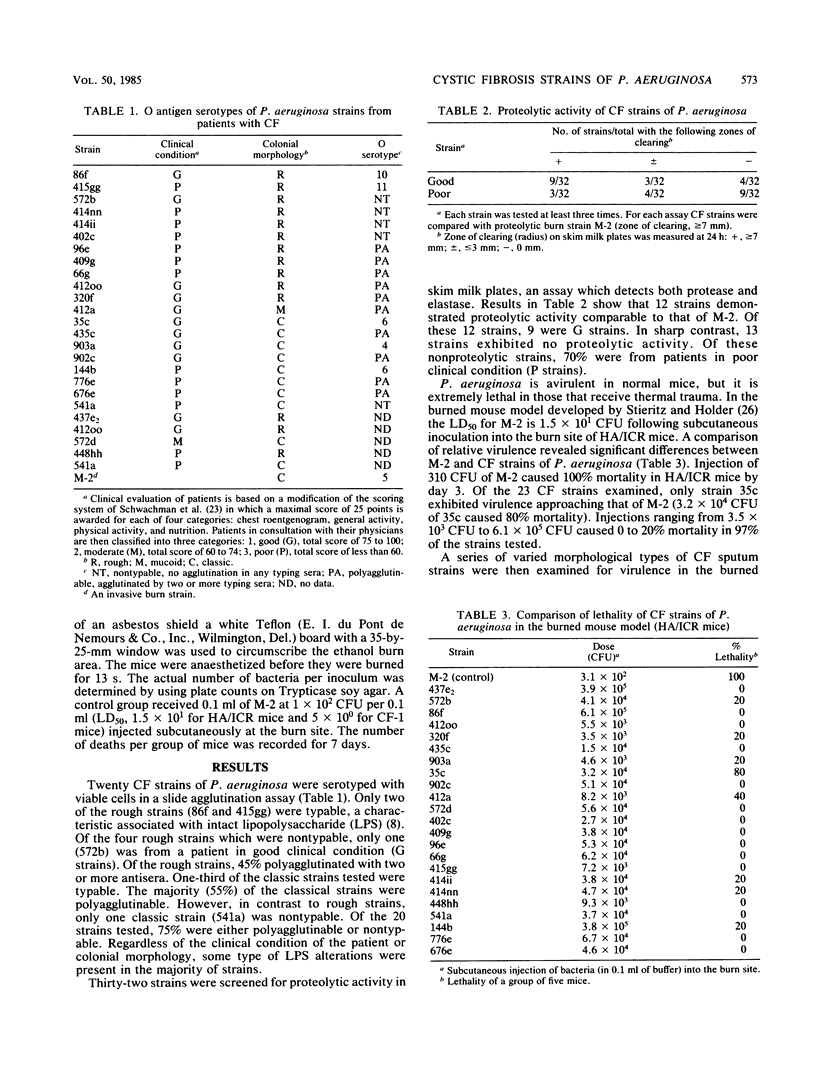
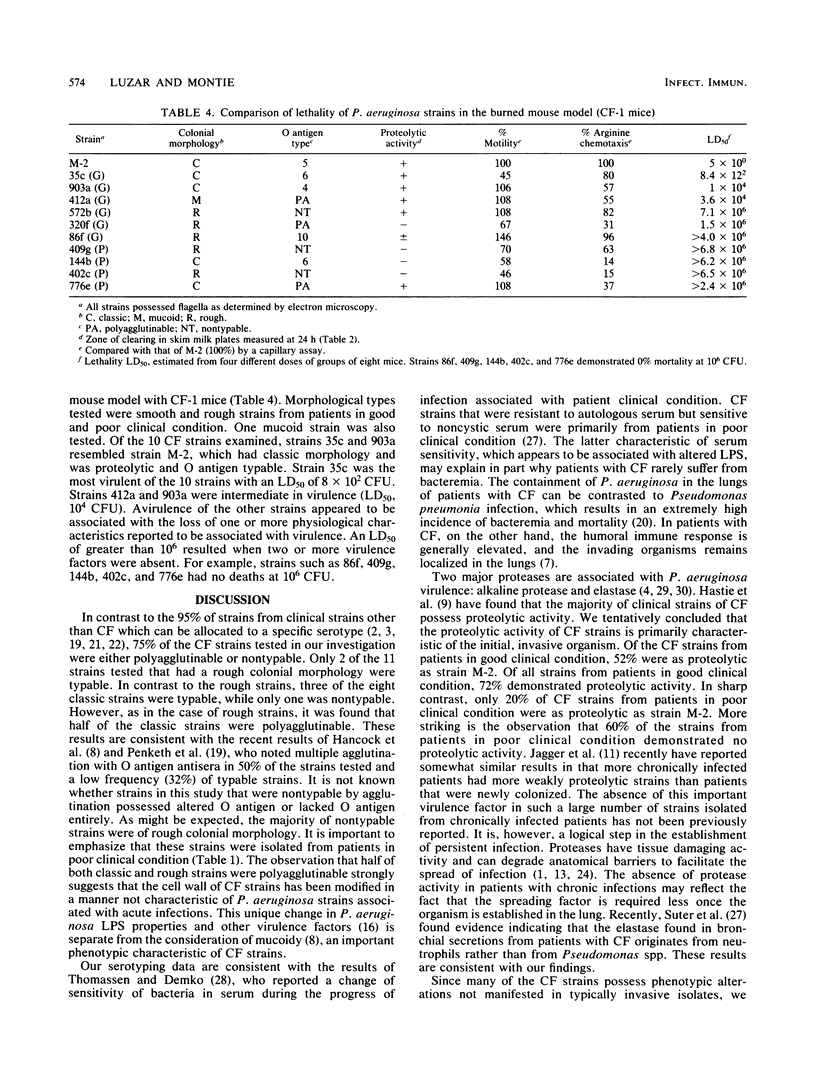
Twenty Pseudomonas aeruginosa strains isolated from patients with cystic fibrosis in good and poor clinical condition were typed by the American Scientific (Difco Laboratories, Detroit, Mich.) Typing Scheme. Only five strains were agglutinated with a single typing serum. Ten strains were agglutinated with more than one serum, and five were not agglutinated with any serum, suggesting some type of lipopolysaccharide alteration in the majority of these strains. Of the strains from patients in good clinical condition, 72% demonstrated proteolytic activity, while 60% of the strains from patients in poor clinical condition demonstrated no proteolytic activity. Twenty-three cystic fibrosis strains of P. aeruginosa examined demonstrated reduced bacteremic virulence when compared with a virulent burn strain with a 50% lethal dose (LD50) of 1.5 X 10(1) CFU in an invasive burned mouse model. Ninety-two percent of the strains tested were avirulent at doses of 10(3) to 10(5) CFU. The LD50s were determined for 10 selected strains which exhibited specific important morphological and physiological deficiencies. Five of the strains tested gave LD50s greater than 10(6) CFU. Reduced virulence of these strains was associated with loss of two or more physiological characteristics associated with virulence. The cystic fibrosis strains of P. aeruginosa which morphologically and physiologically resembled the virulent burn strain were the most virulent (LD50s of 10(2) to 10(4). Results suggest that some degree of virulence is associated only with classic strains prevalent in early infections. The data suggest that a selection transition occurs in the lungs of patients with cystic fibrosis that favors P. aeruginosa avirulence. The avirulent state may be caused by alterations in the cell envelope, including associated factors such as motility and chemotaxis and protease production.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker N. R. Role of exotoxin A and proteases of Pseudomonas aeruginosa in respiratory tract infections. Can J Microbiol. 1982 Feb;28(2):248–255. doi: 10.1139/m82-033. [DOI] [PubMed] [Google Scholar]
- Brokopp C. D., Gomez-Lus R., Farmer J. J., 3rd Serological typing of Pseudomonas aeruginosa: use of commercial antisera and live antigens. J Clin Microbiol. 1977 Jun;5(6):640–649. doi: 10.1128/jcm.5.6.640-649.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester I. R., Meadow P. M., Pitt T. L. The relationship between the O-antigenic lipopolysaccharides and serological specificity in strains of Pseudomonas aeruginosa of different O-serotypes. J Gen Microbiol. 1973 Oct;78(2):305–318. doi: 10.1099/00221287-78-2-305. [DOI] [PubMed] [Google Scholar]
- Cicmanec J. F., Holder I. A. Growth of Pseudomonas aeruginosa in normal and burned skin extract: role of extracellular proteases. Infect Immun. 1979 Aug;25(2):477–483. doi: 10.1128/iai.25.2.477-483.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Pitt T. L., Fürer E., Germanier R. Role of lipopolysaccharide in virulence of Pseudomonas aeruginosa. Infect Immun. 1984 May;44(2):508–513. doi: 10.1128/iai.44.2.508-513.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döring G., Obernesser H. J., Botzenhart K. Extrazelluläre Toxine von Pseudomonas aeruginosa. II. Einwirkung zweier gereinigter Proteasen auf die menschlichen Immunoglobuline IgG, IgA und sekretorisches IgA. Zentralbl Bakteriol A. 1981 Mar;249(1):89–98. [PubMed] [Google Scholar]
- Hancock R. E., Mutharia L. M., Chan L., Darveau R. P., Speert D. P., Pier G. B. Pseudomonas aeruginosa isolates from patients with cystic fibrosis: a class of serum-sensitive, nontypable strains deficient in lipopolysaccharide O side chains. Infect Immun. 1983 Oct;42(1):170–177. doi: 10.1128/iai.42.1.170-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie A. T., Hingley S. T., Kueppers F., Higgins M. L., Tannenbaum C. S., Weinbaum G. Protease production by Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Infect Immun. 1983 May;40(2):506–513. doi: 10.1128/iai.40.2.506-513.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagger K. S., Bahner D. R., Warren R. L. Protease phenotypes of Pseudomonas aeruginosa isolated from patients with cystic fibrosis. J Clin Microbiol. 1983 Jan;17(1):55–59. doi: 10.1128/jcm.17.1.55-59.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaharajo K., Homma J. Y., Aoyama Y., Morihara K. In vivo studies on protease and elastase from Pseudomonas aeruginosa. Jpn J Exp Med. 1975 Apr;45(2):89–100. [PubMed] [Google Scholar]
- Laraya-Cuasay L. R., Cundy K. R., Huang N. N. Pseudomonas carrier rates of patients with cystic fibrosis and of members of their families. J Pediatr. 1976 Jul;89(1):23–26. doi: 10.1016/s0022-3476(76)80920-1. [DOI] [PubMed] [Google Scholar]
- Lindberg U., Hanson L. A., Jodal U., Lidin-Janson G., Lincoln K., Olling S. Asymptomatic bacteriuria in schoolgirls. II. Differences in escherichia coli causing asymptomatic bacteriuria. Acta Paediatr Scand. 1975 May;64(3):432–436. doi: 10.1111/j.1651-2227.1975.tb03860.x. [DOI] [PubMed] [Google Scholar]
- Luzar M. A., Thomassen M. J., Montie T. C. Flagella and motility alterations in Pseudomonas aeruginosa strains from patients with cystic fibrosis: relationship to patient clinical condition. Infect Immun. 1985 Nov;50(2):577–582. doi: 10.1128/iai.50.2.577-582.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May J. R., Herrick N. C., Thompson D. Bacterial infection in cystic fibrosis. Arch Dis Child. 1972 Dec;47(256):908–913. doi: 10.1136/adc.47.256.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montie T. C., Doyle-Huntzinger D., Craven R. C., Holder I. A. Loss of virulence associated with absence of flagellum in an isogenic mutant of Pseudomonas aeruginosa in the burned-mouse model. Infect Immun. 1982 Dec;38(3):1296–1298. doi: 10.1128/iai.38.3.1296-1298.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penketh A., Pitt T., Roberts D., Hodson M. E., Batten J. C. The relationship of phenotype changes in Pseudomonas aeruginosa to the clinical condition of patients with cystic fibrosis. Am Rev Respir Dis. 1983 May;127(5):605–608. doi: 10.1164/arrd.1983.127.5.605. [DOI] [PubMed] [Google Scholar]
- Phair J. P., Bassaris H. P., Williams J. E., Metzger E. Bacteremic pneumonia due to gram-negative bacilli. Arch Intern Med. 1983 Nov;143(11):2147–2149. [PubMed] [Google Scholar]
- Pitt T. L., Erdman Y. J. The specificity of agglutination reactions of Pseudomonas aeruginosa with O antisera. J Med Microbiol. 1978 Feb;11(1):15–23. doi: 10.1099/00222615-11-1-15. [DOI] [PubMed] [Google Scholar]
- Pitt T. L. State of the art: typing Pseudomonas aeruginosa. J Hosp Infect. 1980 Sep;1(3):193–199. doi: 10.1016/0195-6701(80)90056-0. [DOI] [PubMed] [Google Scholar]
- Shwachman H., Kulczycki L. L., Khaw K. T. A report on sixty-five patients over 17 years of age. Pediatrics. 1965 Nov;36(5):689–699. [PubMed] [Google Scholar]
- Snell K., Holder I. A., Leppla S. A., Saelinger C. B. Role of exotoxin and protease as possible virulence factors in experimental infections with Pseudomonas aeruginosa. Infect Immun. 1978 Mar;19(3):839–845. doi: 10.1128/iai.19.3.839-845.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol P. A., Ohman D. E., Iglewski B. H. A more sensitive plate assay for detection of protease production by Pseudomanas aeruginosa. J Clin Microbiol. 1979 Apr;9(4):538–540. doi: 10.1128/jcm.9.4.538-540.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieritz D. D., Holder I. A. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: description of a burned mouse model. J Infect Dis. 1975 Jun;131(6):688–691. doi: 10.1093/infdis/131.6.688. [DOI] [PubMed] [Google Scholar]
- Suter S., Schaad U. B., Roux L., Nydegger U. E., Waldvogel F. A. Granulocyte neutral proteases and Pseudomonas elastase as possible causes of airway damage in patients with cystic fibrosis. J Infect Dis. 1984 Apr;149(4):523–531. doi: 10.1093/infdis/149.4.523. [DOI] [PubMed] [Google Scholar]
- Thomassen M. J., Demko C. A. Serum bactericidal effect on Pseudomonas aeruginosa isolates from cystic fibrosis patients. Infect Immun. 1981 Aug;33(2):512–518. doi: 10.1128/iai.33.2.512-518.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Cryz S. J., Friedman R. L., Iglewski B. H. Contribution of toxin A and elastase to virulence of Pseudomonas aeruginosa in chronic lung infections of rats. Infect Immun. 1982 Jun;36(3):1223–1228. doi: 10.1128/iai.36.3.1223-1228.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wretlind B., Pavlovskis O. R. The role of proteases and exotoxin A in the pathogenicity of Pseudomonas aeruginosa infections. Scand J Infect Dis Suppl. 1981;29:13–19. [PubMed] [Google Scholar]
- di Sant'Agnese P. A., Davis P. B. Research in cystic fibrosis (third of three parts). N Engl J Med. 1976 Sep 9;295(11):597–602. doi: 10.1056/NEJM197609092951105. [DOI] [PubMed] [Google Scholar]


