Abstract
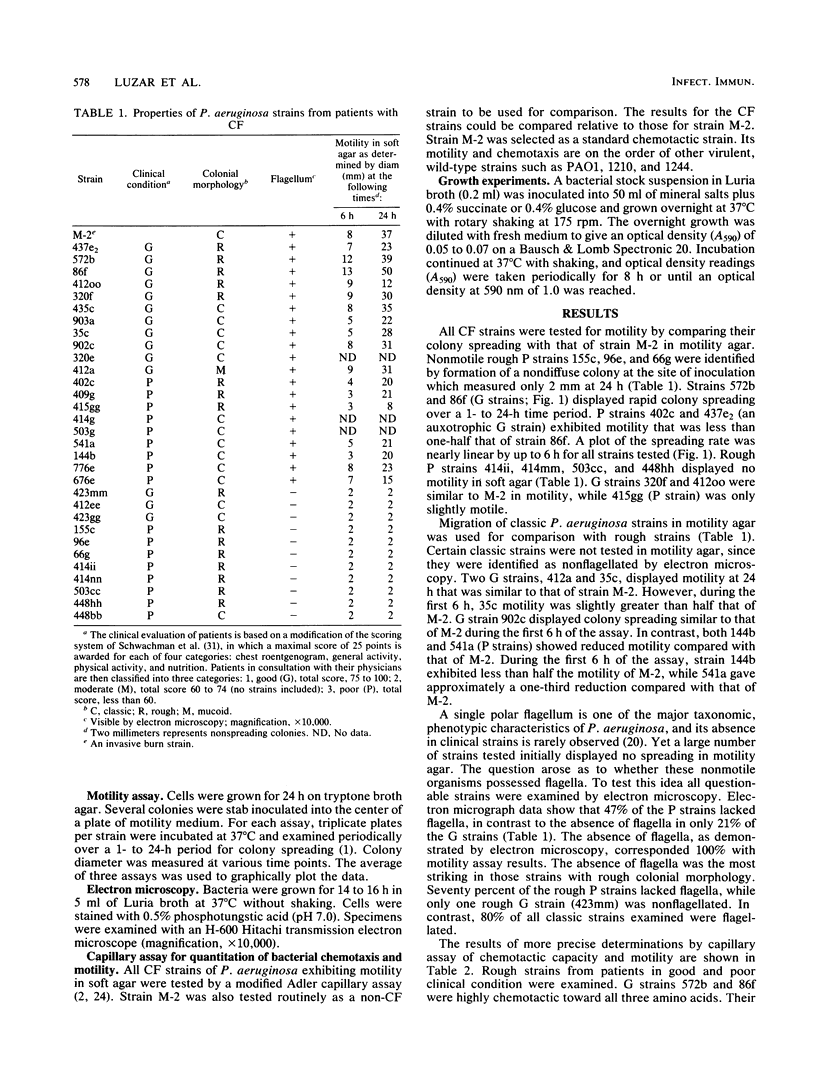
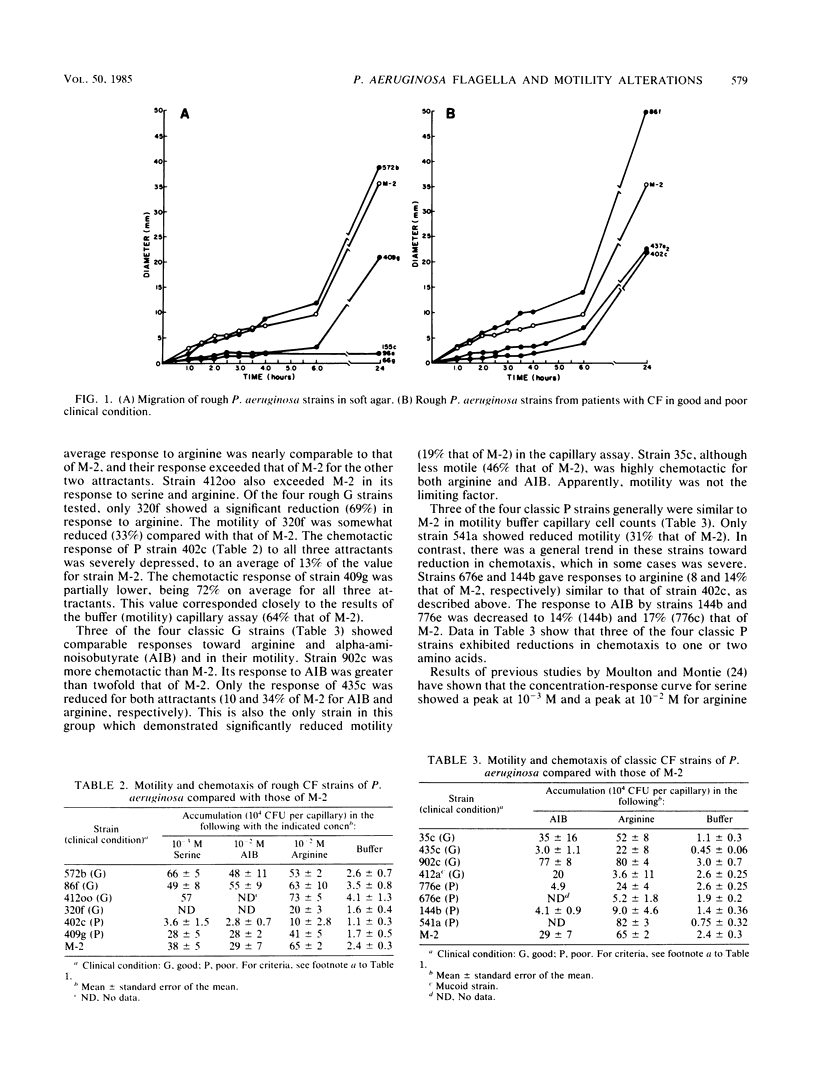
Selected physiological parameters of 31 classic and rough Pseudomonas aeruginosa strains from respiratory tract cultures of patients with cystic fibrosis were examined. An association of a patient's clinical condition (good or poor) with strain physiology was made. Rough strains from patients in poor clinical condition demonstrated severe alterations in motility when compared with M-2, a highly motile and chemotactic burn strain. Of the 10 rough strains from patients in poor clinical condition, 70% lacked flagella, as determined by electron microscopy. The remaining few flagellated strains from this group exhibited weak motility both in soft agar and by the capillary assay. Their chemotactic response to three amino acids, when compared with that of strain M-2, was reduced approximately 30 to 90%. Classic strains from patients in poor clinical condition were less chemotactic than those from patients in good clinical condition. A majority of classic and rough strains from patients in good clinical condition were comparable to M-2 in both chemotaxis and motility. Changes in other physiological characteristics indicated by reduced growth rates, or auxotrophy, were seldom observed in the cystic fibrosis strains studied. The data suggest that host-selective pressures, associated primarily with patients with cystic fibrosis that are in poor clinical condition, result in the loss of factors related to invasiveness such as motility and chemotaxis. We propose that these results may reflect that there is a more general alteration in the cell envelope of cystic fibrosis strains.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973 Jan;74(1):77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- Adler J. Chemotaxis in bacteria. Science. 1966 Aug 12;153(3737):708–716. doi: 10.1126/science.153.3737.708. [DOI] [PubMed] [Google Scholar]
- Ansorg R. Flagellaspezifisches H-Antigenschema von Pseudomonas aeruginosa. Zentralbl Bakteriol Orig A. 1978 Nov;242(2):228–238. [PubMed] [Google Scholar]
- Boyce J. R., Miller R. V. Motility as a selective force in the reversion of cystic fibrosis-associated mucoid Pseudomonas aeruginosa to the nonmucoid phenotype in culture. Infect Immun. 1982 Aug;37(2):840–844. doi: 10.1128/iai.37.2.840-844.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce J. R., Miller R. V. Selection of nonmucoid derivatives of mucoid Pseudomonas aeruginosa is strongly influenced by the level of iron in the culture medium. Infect Immun. 1982 Aug;37(2):695–701. doi: 10.1128/iai.37.2.695-701.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven R. C., Montie T. C. Motility and chemotaxis of three strains of Pseudomonas aeruginosa used for virulence studies. Can J Microbiol. 1981 Apr;27(4):458–460. doi: 10.1139/m81-070. [DOI] [PubMed] [Google Scholar]
- Govan J. R., Fyfe J. A., McMillan C. The instability of mucoid Pseudomonas aeruginosa: fluctuation test and improved stability of the mucoid form in shaken culture. J Gen Microbiol. 1979 Jan;110(1):229–232. doi: 10.1099/00221287-110-1-229. [DOI] [PubMed] [Google Scholar]
- Govan J. R., Fyfe J. A. Mucoid Pseudomonas aeruginosa and cystic fibrosis: resistance of the mucoid from to carbenicillin, flucloxacillin and tobramycin and the isolation of mucoid variants in vitro. J Antimicrob Chemother. 1978 May;4(3):233–240. doi: 10.1093/jac/4.3.233. [DOI] [PubMed] [Google Scholar]
- Govan J. R. Mucoid strains of Pseudomonas aeruginosa: the influence of culture medium on the stability of mucus production. J Med Microbiol. 1975 Nov;8(4):513–522. doi: 10.1099/00222615-8-4-513. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Mutharia L. M., Chan L., Darveau R. P., Speert D. P., Pier G. B. Pseudomonas aeruginosa isolates from patients with cystic fibrosis: a class of serum-sensitive, nontypable strains deficient in lipopolysaccharide O side chains. Infect Immun. 1983 Oct;42(1):170–177. doi: 10.1128/iai.42.1.170-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiby N. Pseudomonas aeruginosa infection in cystic fibrosis. Relationship between mucoid strains of Pseudomonas aeruginosa and the humoral immune response. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Aug;82(4):551–558. [PubMed] [Google Scholar]
- Holder I. A., Wheeler R., Montie T. C. Flagellar preparations from Pseudomonas aeruginosa: animal protection studies. Infect Immun. 1982 Jan;35(1):276–280. doi: 10.1128/iai.35.1.276-280.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaharajo K., Homma J. Y., Aoyama Y., Morihara K. In vivo studies on protease and elastase from Pseudomonas aeruginosa. Jpn J Exp Med. 1975 Apr;45(2):89–100. [PubMed] [Google Scholar]
- Komeda Y., Icho T., Iino T. Effects of galU mutation on flagellar formation in Escherichia coli. J Bacteriol. 1977 Feb;129(2):908–915. doi: 10.1128/jb.129.2.908-915.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koplow J., Goldfine H. Alterations in the outer membrane of the cell envelope of heptose-deficient mutants of Escherichia coli. J Bacteriol. 1974 Feb;117(2):527–543. doi: 10.1128/jb.117.2.527-543.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J., Chan R., Lam K., Costerton J. W. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect Immun. 1980 May;28(2):546–556. doi: 10.1128/iai.28.2.546-556.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laraya-Cuasay L. R., Cundy K. R., Huang N. N. Pseudomonas carrier rates of patients with cystic fibrosis and of members of their families. J Pediatr. 1976 Jul;89(1):23–26. doi: 10.1016/s0022-3476(76)80920-1. [DOI] [PubMed] [Google Scholar]
- Luzar M. A., Montie T. C. Avirulence and altered physiological properties of cystic fibrosis strains of Pseudomonas aeruginosa. Infect Immun. 1985 Nov;50(2):572–576. doi: 10.1128/iai.50.2.572-576.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lányi B. Serological properties of Pseudomonas aeruginosa. II. Type-specific thermolabile (flagellar) antigens. Acta Microbiol Acad Sci Hung. 1970;17(1):35–48. [PubMed] [Google Scholar]
- May J. R., Herrick N. C., Thompson D. Bacterial infection in cystic fibrosis. Arch Dis Child. 1972 Dec;47(256):908–913. doi: 10.1136/adc.47.256.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton R. C., Montie T. C. Chemotaxis by Pseudomonas aeruginosa. J Bacteriol. 1979 Jan;137(1):274–280. doi: 10.1128/jb.137.1.274-280.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penketh A., Pitt T., Roberts D., Hodson M. E., Batten J. C. The relationship of phenotype changes in Pseudomonas aeruginosa to the clinical condition of patients with cystic fibrosis. Am Rev Respir Dis. 1983 May;127(5):605–608. doi: 10.1164/arrd.1983.127.5.605. [DOI] [PubMed] [Google Scholar]
- Pugashetti B. K., Metzger H. M., Cardamone J. J., Edwards D. M., Feingold D. S. Flagellation is a strain-dependent characteristic of cystic fibrosis-associated mucoid strains of Pseudomonas aeruginosa. Eur J Clin Microbiol. 1983 Aug;2(4):360–361. doi: 10.1007/BF02019471. [DOI] [PubMed] [Google Scholar]
- Reynolds H. Y., Di Sant'Agnese P. A., Zierdt C. H. Mucoid Pseudomonas aeruginosa. A sign of cystic fibrosis in young adults with chronic pulmonary disease? JAMA. 1976 Nov 8;236(19):2190–2192. doi: 10.1001/jama.236.19.2190. [DOI] [PubMed] [Google Scholar]
- Seale T. W., Thirkill H., Tarpay M., Flux M., Rennert O. M. Serotypes and antibiotic susceptibilities of Pseudomonas aeruginosa isolates from single sputa of cystic fibrosis patients. J Clin Microbiol. 1979 Jan;9(1):72–78. doi: 10.1128/jcm.9.1.72-78.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shwachman H., Kulczycki L. L., Khaw K. T. A report on sixty-five patients over 17 years of age. Pediatrics. 1965 Nov;36(5):689–699. [PubMed] [Google Scholar]
- Snell K., Holder I. A., Leppla S. A., Saelinger C. B. Role of exotoxin and protease as possible virulence factors in experimental infections with Pseudomonas aeruginosa. Infect Immun. 1978 Mar;19(3):839–845. doi: 10.1128/iai.19.3.839-845.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford B. C., Dixson S., Cobcroft A. J. Serum levels of gentamicin and tobramycin after slow intravenous bolus injection. Lancet. 1974 Mar 9;1(7854):378–379. doi: 10.1016/s0140-6736(74)93148-1. [DOI] [PubMed] [Google Scholar]
- Thomassen M. J., Demko C. A. Serum bactericidal effect on Pseudomonas aeruginosa isolates from cystic fibrosis patients. Infect Immun. 1981 Aug;33(2):512–518. doi: 10.1128/iai.33.2.512-518.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M., Iino T. Ordering of the flagellar genes in Pseudomonas aeruginosa by insertions of mercury transposon Tn501. J Bacteriol. 1983 Feb;153(2):1008–1017. doi: 10.1128/jb.153.2.1008-1017.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M., Iino T. Transductional analysis of the flagellar genes in Pseudomonas aeruginosa. J Bacteriol. 1983 Feb;153(2):1018–1026. doi: 10.1128/jb.153.2.1018-1026.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAHBA A. H., DARRELL J. H. THE IDENTIFICATION OF ATYPICAL STRAINS OF PSEUDOMONAS AERUGINOSA. J Gen Microbiol. 1965 Mar;38:329–342. doi: 10.1099/00221287-38-3-329. [DOI] [PubMed] [Google Scholar]
- Wood R. E., Boat T. F., Doershuk C. F. Cystic fibrosis. Am Rev Respir Dis. 1976 Jun;113(6):833–878. doi: 10.1164/arrd.1976.113.6.833. [DOI] [PubMed] [Google Scholar]
- di Sant'Agnese P. A., Davis P. B. Research in cystic fibrosis (third of three parts). N Engl J Med. 1976 Sep 9;295(11):597–602. doi: 10.1056/NEJM197609092951105. [DOI] [PubMed] [Google Scholar]



