Abstract
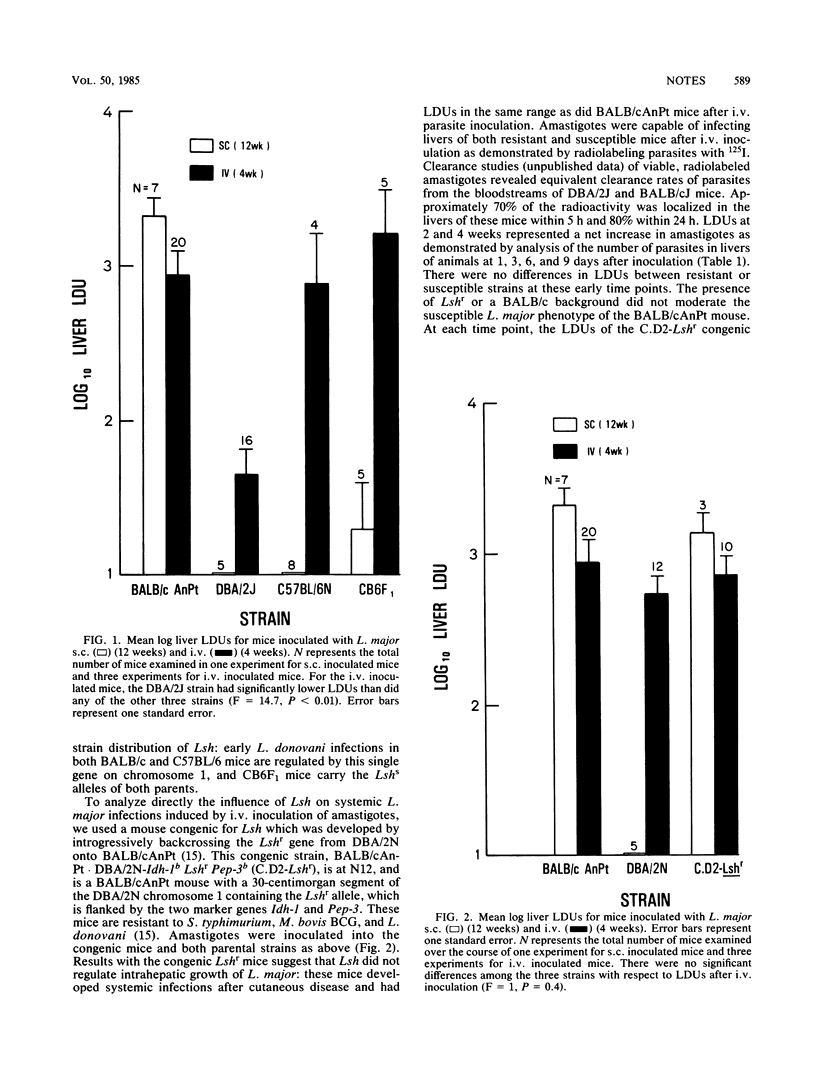
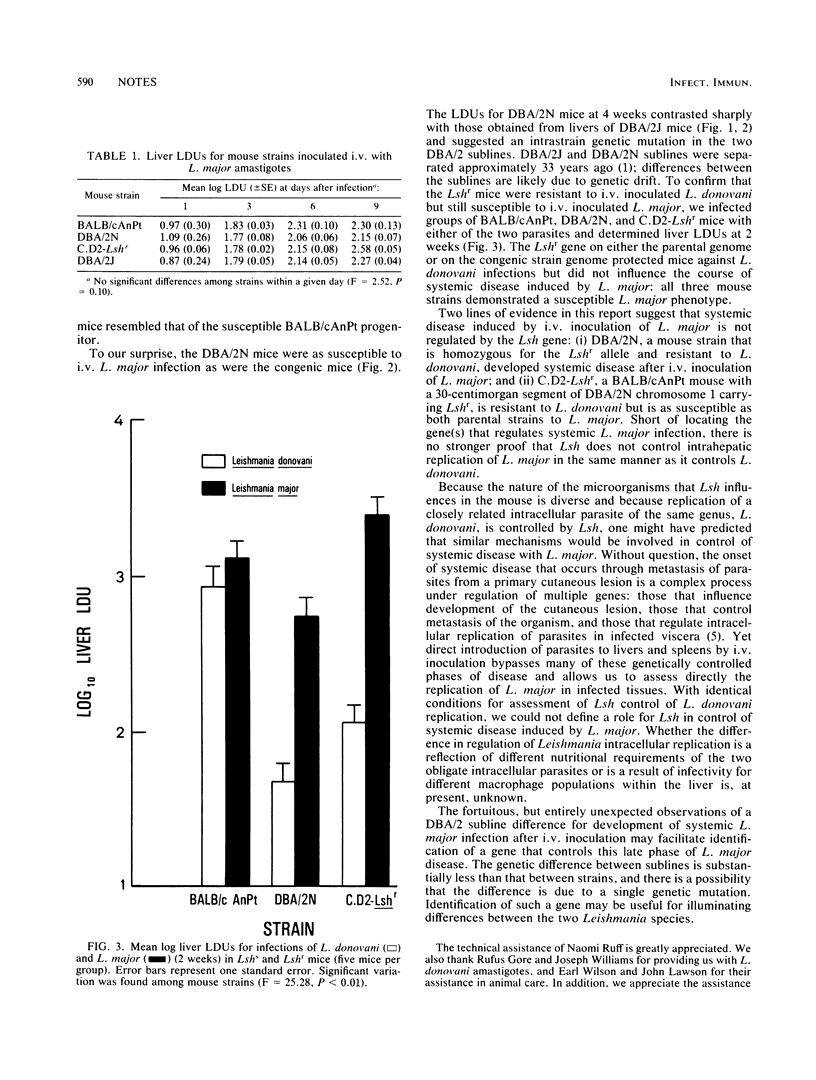
Systemic disease induced by Leishmania major was estimated by microscopic examination of liver impression smears and determination of numbers of intrahepatic amastigotes in intravenously or subcutaneously infected inbred, hybrid, and congenic mice. The distribution of susceptible phenotypes among these mice, particularly the susceptibility of a strain congenic for Lshr, strongly suggested that Lsh, a gene which controls intrahepatic replication of Leishmania donovani, does not influence systemic disease by L. major.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley D. J. Regulation of Leishmania populations within the host. II. genetic control of acute susceptibility of mice to Leishmania donovani infection. Clin Exp Immunol. 1977 Oct;30(1):130–140. [PMC free article] [PubMed] [Google Scholar]
- Bradley D. J., Taylor B. A., Blackwell J., Evans E. P., Freeman J. Regulation of Leishmania populations within the host. III. Mapping of the locus controlling susceptibility to visceral leishmaniasis in the mouse. Clin Exp Immunol. 1979 Jul;37(1):7–14. [PMC free article] [PubMed] [Google Scholar]
- Crocker P. R., Blackwell J. M., Bradley D. J. Expression of the natural resistance gene Lsh in resident liver macrophages. Infect Immun. 1984 Mar;43(3):1033–1040. doi: 10.1128/iai.43.3.1033-1040.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier A. H., Meltzer M. S., Nacy C. A. Susceptibility of inbred mice to Leishmania tropica infection: genetic control of the development of cutaneous lesions in P/J mice. J Immunol. 1984 Jul;133(1):454–459. [PubMed] [Google Scholar]
- Goto Y., Nakamura R. M., Takahashi H., Tokunaga T. Genetic control of resistance to Mycobacterium intracellulare infection in mice. Infect Immun. 1984 Oct;46(1):135–140. doi: 10.1128/iai.46.1.135-140.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros P., Skamene E., Forget A. Genetic control of natural resistance to Mycobacterium bovis (BCG) in mice. J Immunol. 1981 Dec;127(6):2417–2421. [PubMed] [Google Scholar]
- Hockmeyer W. T., Walters D., Gore R. W., Williams J. S., Fortier A. H., Nacy C. A. Intracellular destruction of Leishmania donovani and Leishmania tropica amastigotes by activated macrophages: dissociation of these microbicidal effector activities in vitro. J Immunol. 1984 Jun;132(6):3120–3125. [PubMed] [Google Scholar]
- Jackson P. R., Pappas M. G., Hansen B. D. Fluorogenic substrate detection of viable intracellular and extracellular pathogenic protozoa. Science. 1985 Jan 25;227(4685):435–438. doi: 10.1126/science.2578226. [DOI] [PubMed] [Google Scholar]
- Lissner C. R., Swanson R. N., O'Brien A. D. Genetic control of the innate resistance of mice to Salmonella typhimurium: expression of the Ity gene in peritoneal and splenic macrophages isolated in vitro. J Immunol. 1983 Dec;131(6):3006–3013. [PubMed] [Google Scholar]
- Nacy C. A., Fortier A. H., Pappas M. G., Henry R. R. Susceptibility of inbred mice to Leishmania tropica infection: correlation of susceptibility with in vitro defective macrophage microbicidal activities. Cell Immunol. 1983 Apr 15;77(2):298–307. doi: 10.1016/0008-8749(83)90030-8. [DOI] [PubMed] [Google Scholar]
- Plant J. E., Blackwell J. M., O'Brien A. D., Bradley D. J., Glynn A. A. Are the Lsh and Ity disease resistance genes at one locus on mouse chromosome 1? Nature. 1982 Jun 10;297(5866):510–511. doi: 10.1038/297510a0. [DOI] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Genetics of resistance to infection with Salmonella typhimurium in mice. J Infect Dis. 1976 Jan;133(1):72–78. doi: 10.1093/infdis/133.1.72. [DOI] [PubMed] [Google Scholar]
- Potter M., O'Brien A. D., Skamene E., Gros P., Forget A., Kongshavn P. A., Wax J. S. A BALB/c congenic strain of mice that carries a genetic locus (Ityr) controlling resistance to intracellular parasites. Infect Immun. 1983 Jun;40(3):1234–1235. doi: 10.1128/iai.40.3.1234-1235.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P. A., Farrell J. P. Experimental cutaneous leishmaniasis: disseminated leishmaniasis in genetically susceptible and resistant mice. Am J Trop Med Hyg. 1982 Mar;31(2):230–238. doi: 10.4269/ajtmh.1982.31.230. [DOI] [PubMed] [Google Scholar]
- Skamene E., Gros P., Forget A., Kongshavn P. A., St Charles C., Taylor B. A. Genetic regulation of resistance to intracellular pathogens. Nature. 1982 Jun 10;297(5866):506–509. doi: 10.1038/297506a0. [DOI] [PubMed] [Google Scholar]
- Stach J. L., Gros P., Forget A., Skamene E. Phenotypic expression of genetically-controlled natural resistance to Mycobacterium bovis (BCG). J Immunol. 1984 Feb;132(2):888–892. [PubMed] [Google Scholar]
- Taylor B. A., O'Brien A. D. Position on mouse chromosome 1 of a gene that controls resistance to Salmonella typhimurium. Infect Immun. 1982 Jun;36(3):1257–1260. doi: 10.1128/iai.36.3.1257-1260.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]



