Abstract
Our understanding of the basic biology of diabetes has been guided by observations made using animal models, particularly rodents. However, humans are not mice, and outcomes predicted by murine studies are not always representative of actual outcomes in the clinic. In particular, investigators studying diabetes have relied heavily on mouse and rat models of autoimmune type 1-like diabetes, and experimental results using these models have not been representative of many of the clinical trials in type 1 diabetes. In this manuscript, we describe the availability of new models of humanized mice for the study of three areas of diabetes. These include the use of humanized mice for the study of 1) human islet stem and progenitor cells, 2) human islet allograft rejection, and 3) human immunity and autoimmunity. These humanized mouse models provide an important pre-clinical bridge between in vitro studies and rodent models and the translation of discoveries in these model systems to the clinic.
Keywords: Humanized mouse, Diabetes, Beta cell function
INTRODUCTION
Our understanding of the basic biology of diabetes and autoimmunity has been guided by observations made using animal models, particularly rodents. In animal models, the genetics controlling immune responses can be deliberately altered, genes can be transgenically expressed or mutated, and the level and timing of gene expression can be precisely manipulated. Interventions on cell and tissue function can be readily tested in animal models, and ready access to tissues and cells permit in depth analyses. In rodent models, cells and tissues can be purposefully manipulated in ways that simply cannot be performed in humans. However, mice are not humans, and outcomes predicted by murine studies are not always representative of actual outcomes in the clinic, particularly in trials for prevention or treatment of type 1 diabetes based on animal models1.
To overcome these limitations, we and others have focused on the development and study of “humanized” micefor review, see 2. Operationally, we define humanized mice as mice engrafted with functional human cells or tissues, or mice expressing human transgenes. Humanized mice permit the in vivo investigation of human cells, tissues, and immune systems without putting patients at risk.
The original observation that immunodeficient mice could be engrafted with human tissues was based on genetically athymic mice with a mutation in the Foxn1 gene (abbreviated nude)3. In 1983, mice with mutations in the protein kinase, DNA-activated, catalytic polypeptide (Prkdc) gene (abbreviated scid) were discovered4, and shortly thereafter, it was demonstrated that these mice could be engrafted with human hematopoietic cells5,6. Over the last ∼20 years, our group has been genetically modifying immunodeficient mice to increase their capacity to support engraftment with human cells and tissues. A major milestone was achieved in 1995 when we observed that NOD-scid mice, which have numerous defects in innate immunity, supported enhanced engraftment of human hematolymphoid tissues7. These mice rapidly became the “gold standard” for the scientific community using humanized mice7. Many genetic modifications to enhance human lymphohematopoietic cell engraftment of the NOD-scid stock have been performed, including the generation of targeted mutations in the recombination activating gene 1 or 2 (Rag1null and Rag2null), or in the beta 2-microglobulin (B2mnull) gene2. However, these models were limited by short lifespans, the presence of NK cell activity and other facets of innate immunity that decreased engraftment of the human lymphohematopoietic cells.
The recent major advance in the field was the development of immunodeficient mice bearing a targeted mutation in the IL-2 receptor common gamma chain (IL-2rγnull)2. The IL-2rγ chain is required for high affinity signaling through multiple cytokine receptors, including IL-2, IL-4, IL-7, IL-9, IL-15, and IL-212. The inability to signal through these receptors leads to severe defects in both adaptive and innate immunity. There are four stocks of immunodeficient mice that have been described in the literature based on the IL-2rγnull mutation. These immunodeficient strains of IL-2rγnull mice include NOD.cg-PrkdcscidIl2rgtm1Wjll (abbreviated as NOD-scid IL-2rγnull), NOD.cg-PrkdcscidIL-2rgtm1Sug (abbreviated as NOG mice), C.129(cg)-Rag2tm1FwaIL-2rgtm1Sug (abbreviated as BALB/c-Rag2nullIL-2rγnull) and Stock (H2d)-Rag2tm1Fwa IL-2rgtm1Krf (abbreviated as H2d Rag2nullIL-2rγnull)2.
Using these immunodeficient stocks bearing an IL-2rγnull targeted mutation, humanized mice have become important pre-clinical tools for biomedical research in a number of areas. The study of human hematopoiesis, including the study of human hematopoietic stem (HSC) and progenitor cells as defined by their ability to generate multi-lineage hematopoietic cell populations in vivo, has been a key component in studies that have identified and characterized human hematopoietic stem cells. Many human-specific infectious diseases such as HIV, Dengue, Ebola, EBV, and measles can now be studied in animal models. The use of humanized mice has also been a critical driving force behind the identification and characterization of cancer stem cells. The use of humanized mice in this area is redefining the critical tumor cell targets of anti-cancer therapies. The emerging field of regenerative medicine is guided by the ability of human cells and tissues to regenerate cell and tissue function in vivo in humanized mice. Finally, the study of human immune systems can now be modeled in vivo in humanized mice. These uses of humanized mice have recently been reviewed2. In this manuscript, we have focused on the development and characterization of humanized mice based on the NOD-scid IL-2rγnull and NOD-Rag1null IL-2rγnull stocks of mice to address areas of interest in diabetes research.
Humanized mice for the analysis of human islet stem and progenitor cells
The use of immunodeficient mice as recipients of syngeneic, allogeneic, and xenogeneic islet grafts has been ongoing for many years. However, the recent emergence of regenerative medicine has opened the possibility that human beta stem and progenitor cells can be identified, expanded and differentiated in vitro, and used to provide an essentially unlimited supply of human beta cells for transplantation. However, the promise of this approach relies on the ability of the generated beta cells to be glucose responsive and secrete physiological levels of insulin to maintain normoglycemia in diabetic hosts.
The immunodeficient recipient most frequently used for most human islet transplantation studies over the last 10 years has been the NOD-scid mouse induced to become hyperglycemic by injection of the beta cell selective toxin, streptozotocin8. However, this mouse stock has a number of limitations for use in the study of human islet stem and progenitor cells. First, NOD-scid mice develop thymic lymphomas, and most die by ∼6 months of age, precluding long term studies of islet stem and progenitor cell differentiation and function. Second, although decreased, NOD-scid mice retain some NK cell activity7. The stem cell sources for human islets are predominately derived from human embryonic stem cells (ESC), mesenchymal stem cells (MSC), HSC, or intra-pancreatic progenitor cells. These stem and progenitor cell populations, and their progeny, are highly sensitive to NK cell killing9-11. Therefore, studies using NOD-scid mice may lead to “false negatives” due to the duration of the experiment or the sensitivity of the stem and progenitor cells and their progeny to NK cell killing.
Our long-term goal is to determine the ability of human islet stem and progenitor cell populations to develop and function in the presence of an intact human immune system. Human immune system engraftment in NOD-scid mice is variable and not robust due to the variability of human peripheral blood mononuclear cell (PBMC) engraftment or the inability of human HSC to generate functional human T cells in vivo7,11. Furthermore, the induction of hyperglycemia by chemical toxins such as streptozotocin will also severely impair the function of the engrafted human immune system, rendering a chemical approach for the induction of diabetes or transplantation of islets or their precursors not practical.
To address these issues, we are developing the NOD-Rag1null IL-2rγnull Ins2Akita stock of mice. The Akita mutation is an insulin 2 gene defect that leads to generation of mis-folded insulin protein, induction of endoplasmic reticulum stress, and beta cell apoptosis12. A non-autoimmune hyperglycemia will develop in these immunodeficient mice as they age in the absence of toxic chemical treatment that could harm the transplanted human islets or their precursors. We have also found that NOD-Rag1null IL-2rγnull mice, similar to NOD-scid IL-2rγnull mice, engraft at high levels with human HSC and with PBMC that will permit the study of human islets or their precursors in the presence of a robust human immune system (TP, unpublished observations).
Humanized mice for the analysis of human immune systems
For the in vivo study of a human immune system, investigators have developed three human immune system mouse models (Table 1). First, as early as 1988, investigators engrafted immunodeficient mice with human PBMC, termed Hu-PBL-SCID mice5. Second, also in 1988, immunodeficient mice were shown to support engraftment with human HSC6. This model system was established using human fetal liver and thymus (SCID-hu), or human HSC (human Scid Repopulating Cell, or Hu-SRC-SCID)2. More recently, immunocompetent mice have been genetically engineered to express human genes, in particular human HLA genes that permit the identification of the antigenic epitopes that are presented by human HLA genes to the immune system13.
Table 1. Humanized Immune System (HIS) Mouse Models.
Models of human immune system mice for the in vivo study of human immune systems.
| Model | Characteristics | Uses |
|---|---|---|
| PBMC Engraftment | Immunodeficient mice engrafted with human PBMCs | Study of human-specific infectious agents, alloimmunity and autoimmunity in vivo |
| Stem Cell Engraftment | Immunodeficient mice engrafted with human HSCs | Study of human HSC and development of a functional human immune system |
| Human MHC Tg | HLA-Tg mice | Detection of epitopes that are presented by human HLA molecules |
Hu-PBL-SCID Mice
This model is based on the injection of human PBMC into unconditioned immunodeficient mice. Although the original stock of mice, the CB17-scid, could support engraftment of human PBMC, the engraftment was quite low (<1%) and variable both between different donors and among recipients of a single donor7. Although higher engraftment was observed in NOD-scid mice, engraftment continued to be low (1−10%) and remained quite variable. We have recently investigated the engraftment of human PBMC into NOD-scid IL-2rγnull mice14. We addressed a number of parameters, including the optimal recipient, cell dose, route of engraftment, and kinetics of engraftment.
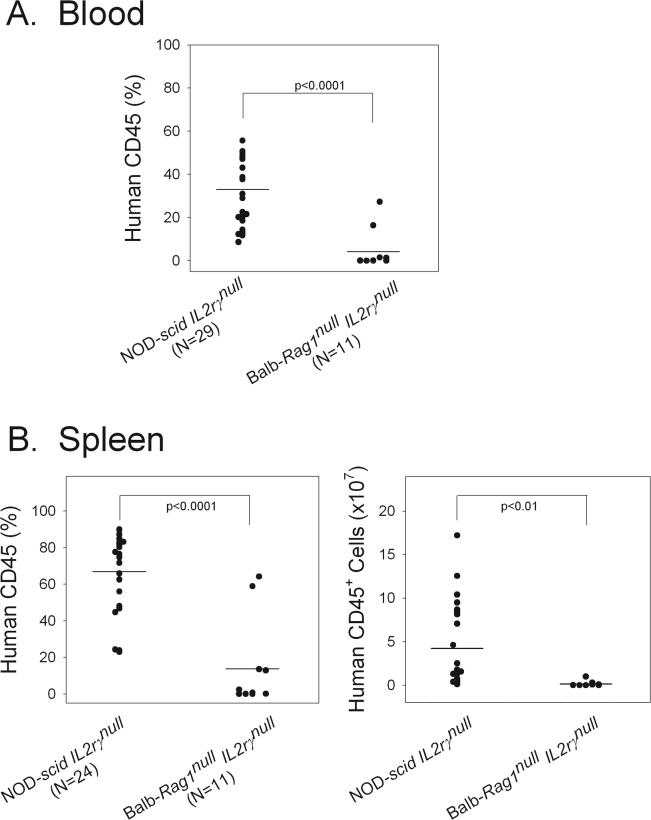
To identify the optimal strain, we generated a new stock of mice, the BALB/c-Rag1null IL-2rγnull (LDS, unpublished observations) stock, and determined the ability of human PBMC to engraft in this stock as compared with NOD-scid Il2rγnull mice (Figure 1). We observed that PBMC engraftment in NOD-scid IL-2rγnull mice was much higher than that achieved in BALB/c-Rag1null IL-2rγnull mice (Figure 1). Based on this observation, we then used the NOD-scid IL-2rγnull stock of mice to determine that, in contrast to previous Hu-PBL-SCID models2, intravenous injection of human PBMC led to high engraftment, and that engraftment could be achieved following intravenous injection with as few as 1−5×106 PBMC. We further observed that all mice engrafted using PBMC doses that were ≥10×106 cells, and that engraftment increased up to 3−4 weeks after injection14. These data documented that immunodeficient NOD-scid IL-2rγnull mice support higher engraftment of human PBMC as compared with the BALB/c-Rag1null IL-2rγnull stock of mice or previous stocks of immunodeficient mice not based on the IL-2rγnull mutation2
Figure 1. Legend: Optimal human PBMC recipient.
Adult mice of the indicated stocks were injected intravenously with 20×106 human PBMC. Four weeks later, the percent of human CD45+ cells in the blood, and the percent and number of human CD45+ cells in the spleen were determined by flow cytometry.
We further asked whether the engrafted human PBMC were functional. To test this, we determined their ability to reject human islet allografts. We observed that chemically diabetic NOD-scid IL-2rγnull mice transplanted with 4000 IEQ of human islets intrasplenically became normoglycemic. However, if the recipients were transplanted with human islets and injected intravenously with 20×106 human allogeneic PBMC, the human islet allografts were rejected within 7−15 days14.
Hu-SRC-SCID Mice
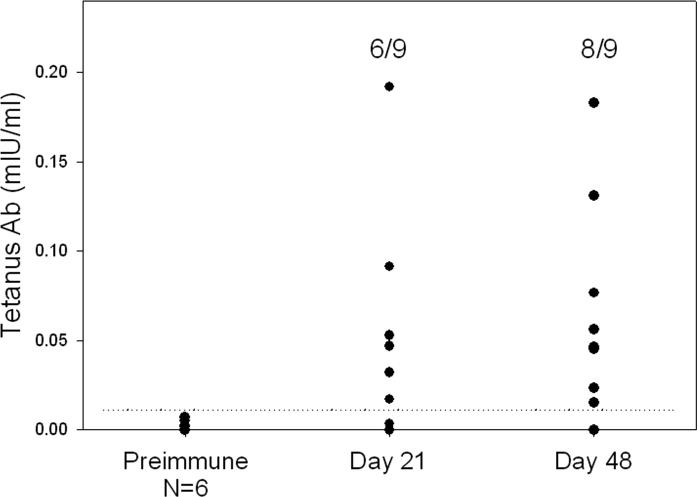
This model system is based on the engraftment of conditioned (irradiated) immunodeficient mice transplanted with human HSC. In this model system, we first optimized the age of the recipient by intravenous injection of newborn and adult NOD-scid IL-2rγnull mice with T cell-depleted umbilical cord blood cells containing 3×104 CD34+ cells. We observed that, although human CD45+ cells develop at comparable levels in both recipients, that T cells were more readily generated in newborn recipients (TP, unpublished observations). We further observed that all human hematolymphoid lineages, including human T cells (CD3, CD4 and CD8), B cells, NK cells, regulatory T cells (CD4+CD25+Foxp3+) and myeloid and plasmacytoid dendritic cells were generated in newborn engrafted mice. Confirming that the engrafted human immune system was functional, we further documented that the Hu-SRC-SCID mice could generate T-dependent antibodies in response to immunization with tetanus toxoid, although the antibody titers were ∼1000-fold lower than that observed in sera from humans immunized to tetanus toxoid (Figure 2).
Figure 2. Legend: Production of T-dependent anti-tetanus antibody following immunization with tetanus toxoid.
Adult NOD-scid IL2rγnull mice were irradiated with 240cGy and injected intravenously with 3×104 CD34+ T cell-depleted umbilical cord blood cells. Approximately 12 weeks later, the engrafted mice were immunized subcutaneously with 150 microliters of tetanus toxoid. Twenty one days later, the mice were bled, and boosted on day 36 with tetanus toxoid, and bled again on day 48. Shown are the anti-tetanus antibody titers at the indicated time points. The dashed line represents 3 standard deviations above the mean value of the tetanus antibody level in prebleeds of immunized mice. Some mice were treated for 4 days with 10μg recombinant BLyS prior to boosting. No differences between BLyS treated and PBS treated mice were observed, and the data have been pooled for presentation.
Humanized mice for the analysis of human autoimmunity
HLA-Tg Immunocompetent Mice
Humanized HLA-transgenic immunocompetent mice have been used for the study of autoimmunity in many model systems, including diabetes13. Of particular interest is the use of immunocompetent HLA-transgenic mice for the identification of autoantigens. For example, HLA-A2-restricted T cells from immunocompetent NOD/Lt HLA-A2.1 transgenic mice have been used to identify islet autoantigens, including epitopes of islet-specific glucose-6-phosphatase catalytic subunit-related gene (IGRP)15 that appear to be an autoimmune target in type 1 diabetic patients16. Furthermore, spontaneous autoimmune diabetes17,18 and multiple sclerosis19 have been observed to develop in HLA-transgenic mice, providing new models for the study of the pathogenesis of autoimmunity.
Hu-PBL-SCID mice
This model system has been used extensively for the study of autoimmunity (Table 2). Investigators have adoptively transferred PBMCs from individuals with many autoimmune disorders, including thyroid disease or diabetes, to immunodeficient mice (Table 2). In recipients of PBMC from diabetic individuals, production of islet-antigen-specific autoantibodies was observed without evidence of insulitis or islet cell damage20. Immunodeficient mice have also been co-transplanted with thyroid organoids and PBMCs from patients with Graves' disease, and the production of thyroid–peroxidase-specific autoantibody was observed21. Comparable studies were done using cells derived from patients with rheumatoid arthritis; scid-mice that received synovial cells or PBMCs from rheumatoid arthritis patients produced antibodies to rheumatoid factor22. However, in all cases, CB17-scid or NOD-scid mice were used as hosts and infiltration and target organ destruction was not observed. This was likely due to low and variable engraftment of human PBMC in CB17-scid mice, and based on our new data, we suggest that even BALB/c-Rag1null IL-2rγnull mice are not optimal hosts for human PBMC (Figure 1). It will be important to optimize these models of human autoimmunity using the new humanized NOD mouse Hu-PBL-SCID model based on the IL-2rγnull mutation as these mice provide more reliable engraftment (Figure 1). These new models might also provide biomarkers of disease progression based on detection of circulating autoreactive T cells or autoantibodies.
Table 2. Hu-PBL-SCID Models of Autoimmunity.
Human autoimmune diseases modeled in humanized Hu-PBL-SCID mice.
| Autoimmune Disease | Target Tissue | Reference |
|---|---|---|
| Type 1 Diabetes | Islets/Beta Cells | 20 |
| Primary Biliary Cirrhosis | Liver | 25 |
| Multiple Sclerosis | Central Nervous System | 19 |
| Autoimmune Thyroid Disease | Thyroid | 21 |
| Rheumatoid Arthritis | Joint Synovium | 22 |
| Psoriasis | Skin | 26 |
| Systemic Lupus Erythematosus | Autoantibodies, multiple targets | 27 |
| Myasthenia Gravis | Acetylcholine Receptors | 28 |
| Autoimmune Hemolytic Anemia | Erythrocytes | 29 |
| Pemphigus Vulgaris | Skin | 30 |
Humanized mice for the analysis of human autoimmunity: The Future
How can humanized mice be used in the future to study human autoimmunity? Based on the ability of human HSC to engraft and generate a functional human immune system, it will be important to determine if HSC derived from a person with a genetic predisposition to autoimmunity will recapitulate autoimmune process in humanized mice. For example, it is known that HSC from NOD mice or BB rats, animal models of type 1 diabetes, can adoptively transfer the disease to naïve recipients23. It has also been documented in the literature that HSC from humans can transfer type 1 diabetes to adoptive recipients24. It will be important to test the ability of human HSC with a known genetic predisposition to type 1 diabetes to generate a human immune system that produces islet autoantibodies, generates autoreactive T cells, and induces insulitis and beta cell destruction. These human HSC could be derived from the bone marrow or mobilized peripheral blood HSC of type 1 diabetic individuals, or from genetically susceptible cord blood HSCs of newborns of type 1 diabetic parents. The availability of standardized islet autoantibody assays as well as tetramers for known islet autoantigens now makes the investigation of the development and function of an autoimmune human immune system in humanized mice possible.
Another approach may be the use of new technology to generate TCR transgenic human immune systems in immunodeficient mice. Human islet autoreactive T cell clones have been generated, and retroviral technology now permits the transduction of human HSC. Coupled with the availability of immunodeficient NOD strains of IL-2rγnull mice that support the generation of a functional human immune system from HSCs, we propose that the generation of human islet autoreactive TCR transgenic humanized mice may now be possible. The development and characterization of these mice could permit the investigation of human autoreactive T cell development and function at various stages of the disease process.
ACKNOWLEDGEMENTS
We thank Linda Paquin, Amy Cuthbert, and Celia Hartigan for their technical assistance. We also thank the Clinical Trials Unit at the University of Massachusetts Medical School. This work was supported by National Institutes of Health Grants AI46629, DK53006, an institutional Diabetes Endocrinology Research Center (DERC) grant DK32520, a Cancer Center Core grant CA34196, the Beta Cell Biology Consortium, and Juvenile Diabetes Foundation, International. Islets used in these experiments were obtained from the JDRF-supported Islet Isolation Center of the University of Pittsburgh or from the Islet Cell Resource Consortium, administered by the ABCC and supported by NCRR, NIDDK and JDRF. TP is supported by a post-doctoral fellowship from the JDRF. MK is supported by a pre-doctoral fellowship from the American Diabetes Association. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.ROEP BO, ATKINSON M. Animal models have little to teach us about Type 1 diabetes: 1. In support of this proposal. Diabetologia. 2004;47:1650–1656. doi: 10.1007/s00125-004-1517-1. [DOI] [PubMed] [Google Scholar]
- 2.SHULTZ LD, ISHIKAWA F, GREINER DL. Humanized mice in translational biomedical research. Nat. Rev Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 3.PANTELOURIS EM. Absence of thymus in a mouse mutant. Nature. 1968;217:370–371. doi: 10.1038/217370a0. [DOI] [PubMed] [Google Scholar]
- 4.BOSMA GC, CUSTER RP, BOSMA MJ. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- 5.MOSIER DE, GULIZIA RJ, BAIRD SM, WILSON DB. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988;335:256–259. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- 6.MCCUNE JM, NAMIKAWA R, KANESHIMA H, SHULTZ LD, LIEBERMAN M, WEISSMAN IL. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 7.GREINER DL, SHULTZ LD. Use of NOD/LtSz-scid/scid mice in biomedical research. In: Leiter EH, Atkinson MA, editors. NOD Mice and Related Strains: Research Applications in Diabetes, AIDS, Cancer and Other Diseases. R.G. Landes Co.; Austin, TX: 1998. pp. 173–204.. [Google Scholar]
- 8.GERLING IC, FRIEDMAN H, GREINER DL, SHULTZ LD, LEITER EH. Multiple low-dose streptozocin-induced diabetes in NOD-scid/scid mice in the absence of functional lymphocytes. Diabetes. 1994;43:433–440. doi: 10.2337/diab.43.3.433. [DOI] [PubMed] [Google Scholar]
- 9.TIAN X, WOLL PS, MORRIS JK, LINEHAN JL, KAUFMAN DS. Hematopoietic engraftment of human embryonic stem cell-derived cells is regulated by recipient innate immunity. Stem Cells. 2006;24:1370–1380. doi: 10.1634/stemcells.2005-0340. [DOI] [PubMed] [Google Scholar]
- 10.SOTIROPOULOU PA, PEREZ SA, GRITZAPIS AD, BAXEVANIS CN, PAPAMICHAIL M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 11.GREINER DL, HESSELTON RA, SHULTZ LD. SCID mouse models of human stem cell engraftment. Stem Cells. 1998;16:166–177. doi: 10.1002/stem.160166. [DOI] [PubMed] [Google Scholar]
- 12.RON D. Proteotoxicity in the endoplasmic reticulum: lessons from the Akita diabetic mouse. J. Clin. Invest. 2002;109:443–445. doi: 10.1172/JCI15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.SERREZE DV, CHEN YG. Of mice and men: use of animal models to identify possible interventions for the prevention of autoimmune type 1 diabetes in humans. Trends Immunol. 2005;26:603–607. doi: 10.1016/j.it.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 14.KING M, PEARSON T, SHULTZ LD, LEIF J, BOTTINO R, TRUCCO M, ATKINSON MA, WASSERFALL C, HEROLD KC, WOODLAND RT, SCHMIDT MR, WODA BA, THOMPSON MJ, ROSSINI AA, GREINER DL. A new Hu-PBL model for the study of human islet alloreactivity based on NOD-scid mice bearing a targeted mutation in the IL-2 receptor gamma chain gene. Clin. Immunol. 2007 doi: 10.1016/j.clim.2007.11.001. In press. [DOI] [PubMed] [Google Scholar]
- 15.TAKAKI T, MARRON MP, MATHEWS CE, GUTTMANN ST, BOTTINO R, TRUCCO M, DILORENZO TP, SERREZE DV. HLA-A*0201-restricted T cells from humanized NOD mice recognize autoantigens of potential clinical relevance to type 1 diabetes. J. Immunol. 2006;176:3257–3265. doi: 10.4049/jimmunol.176.5.3257. [DOI] [PubMed] [Google Scholar]
- 16.SERREZE DV, MARRON MP, DILORENZO TP. “Humanized” HLA transgenic NOD mice to identify pancreatic beta cell autoantigens of potential clinical relevance to type 1 diabetes. Ann. N. Y. Acad. Sci. 2007;1103:103–111. doi: 10.1196/annals.1394.019. [DOI] [PubMed] [Google Scholar]
- 17.WEN L, CHEN NY, TANG J, SHERWIN R, WONG FS. The regulatory role of DR4 in a spontaneous diabetes DQ8 transgenic model. J. Clin. Invest. 2001;107:871–880. doi: 10.1172/JCI11708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MARRON MP, GRASER RT, CHAPMAN HD, SERREZE DV. Functional evidence for the mediation of diabetogenic T cell responses by HLA-A2.1 MHC class I molecules through transgenic expression in NOD mice. Proc. Natl. Acad. Sci. U. S. A. 2002;99:13753–13758. doi: 10.1073/pnas.212221199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ELLMERICH S, MYCKO M, TAKACS K, WALDNER H, WAHID FN, BOYTON RJ, KING RH, SMITH PA, AMOR S, HERLIHY AH, HEWITT RE, JUTTON M, PRICE DA, HAFLER DA, KUCHROO VK, ALTMANN DM. High incidence of spontaneous disease in an HLA-DR15 and TCR transgenic multiple sclerosis model. J. Immunol. 2005;174:1938–1946. doi: 10.4049/jimmunol.174.4.1938. [DOI] [PubMed] [Google Scholar]
- 20.PETERSEN JS, MARSHALL MO, BAEKKESKOV S, HEJNAES KR, HOIER-MADSEN M, DYRBERG T. Transfer of type 1 (insulin-dependent) diabetes mellitus associated autoimmunity to mice with severe combined immunodeficiency (SCID) Diabetologia. 1993;36:510–515. doi: 10.1007/BF02743266. [DOI] [PubMed] [Google Scholar]
- 21.DAVIES TF, KIMURA H, FONG P, KENDLER D, SHULTZ LD, THUNG S, MARTINA The SCID-hu mouse and thyroid autoimmunity: characterization of human thyroid autoantibody secretion. Clin. Immunol. Immunopath. 1991;60:319–330. doi: 10.1016/0090-1229(91)90075-l. [DOI] [PubMed] [Google Scholar]
- 22.RENDT KE, BARRY TS, JONES DM, RICHTER CB, MCCACHREN SS, HAYNES BF. Engraftment of human synovium into severe combined immune deficient mice. Migration of human peripheral blood T cells to engrafted human synovium and to mouse lymph nodes. J. Immunol. 1993;151:7324–7336. [PubMed] [Google Scholar]
- 23.MORDES JP, SERREZE DV, GREINER DL, ROSSINI AA. Animal models of autoimmune diabetes mellitus. In: LeRoith D, Taylor SI, Olefsky JM, editors. Diabetes mellitus. A fundamental and clinical text. Lippincott Williams & Wilkins; Philadelphia: 2004. pp. 591–610. [Google Scholar]
- 24.SORLI CH, GREINER DL, MORDES JP, ROSSINI AA. Stem cell transplantation for treatment of autoimmune diseases. Graft. 1998;1:71–81. [Google Scholar]
- 25.KRAMS SM, DORSHKIND K, GERSHWIN ME. Generation of biliary lesions after transfer of human lymphocytes into severe combined immunodeficient (SCID) mice. J. Exp. Med. 1989;170:1919–1930. doi: 10.1084/jem.170.6.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.IGNEY FH, ASADULLAH K, ZOLLNER TM. Techniques: species' finest blend--humanized mouse models in inflammatory skin disease research. Trends Pharmacol. Sci. 2004;25:543–549. doi: 10.1016/j.tips.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 27.DUCHOSAL MA, MCCONAHEY PJ, ROBINSON CA, DIXON FJ. Transfer of human systemic lupus erythematosus in severe combined immunodeficient (SCID) mice. J. Exp. Med. 1990;172:985–988. doi: 10.1084/jem.172.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MARTINO G, GRIMALDI LM, WOLLMANN RL, BONGIOANNI P, QUINTANS J. The hu-SCID myasthenic mouse. A new tool for the investigation of seronegative myasthenia gravis. Annals of the New York Academy of Sciences. 1993;681:303–305. doi: 10.1111/j.1749-6632.1993.tb22901.x. [DOI] [PubMed] [Google Scholar]
- 29.YATES P, MACHT LM, WILLIAMS NA, ELSON CJ. Red cell autoantibody production by colonic mononuclear cells from a patient with ulcerative colitis and autoimmune haemolytic anaemia. Br. J. Haematol. 1992;82:753–756. doi: 10.1111/j.1365-2141.1992.tb06954.x. [DOI] [PubMed] [Google Scholar]
- 30.JUHASZ I, LAZARUS GS, MURPHY GF, SHIH IM, HERLYN M. Development of pemphigus vulgaris-like lesions in severe combined immunodeficiency disease mice reconstituted with lymphocytes from patients. J. Clin. Invest. 1993;92:2401–2407. doi: 10.1172/JCI116846. [DOI] [PMC free article] [PubMed] [Google Scholar]