Abstract
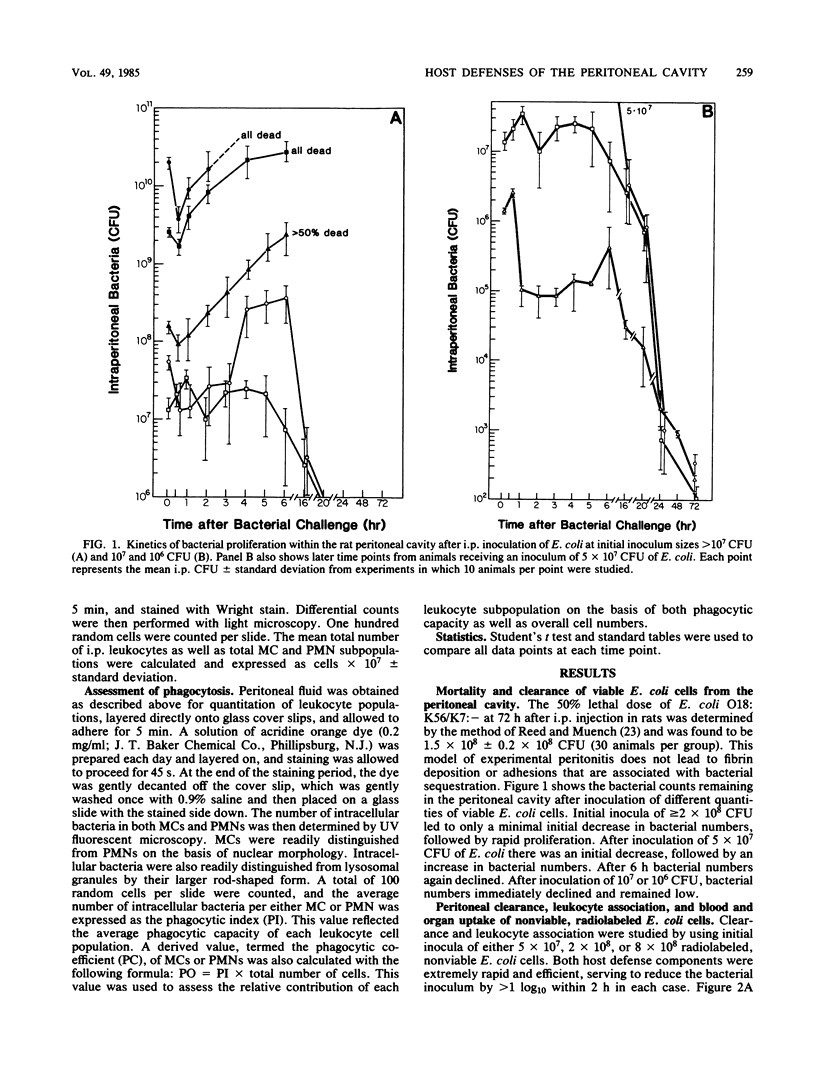
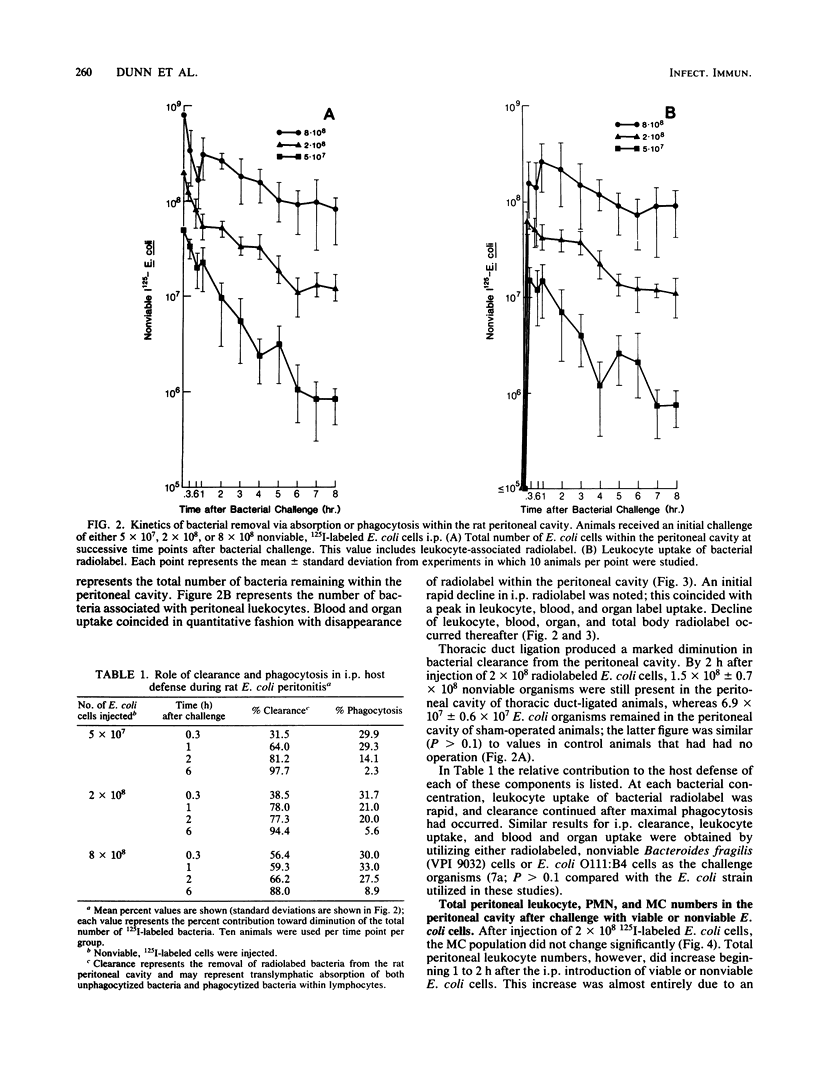
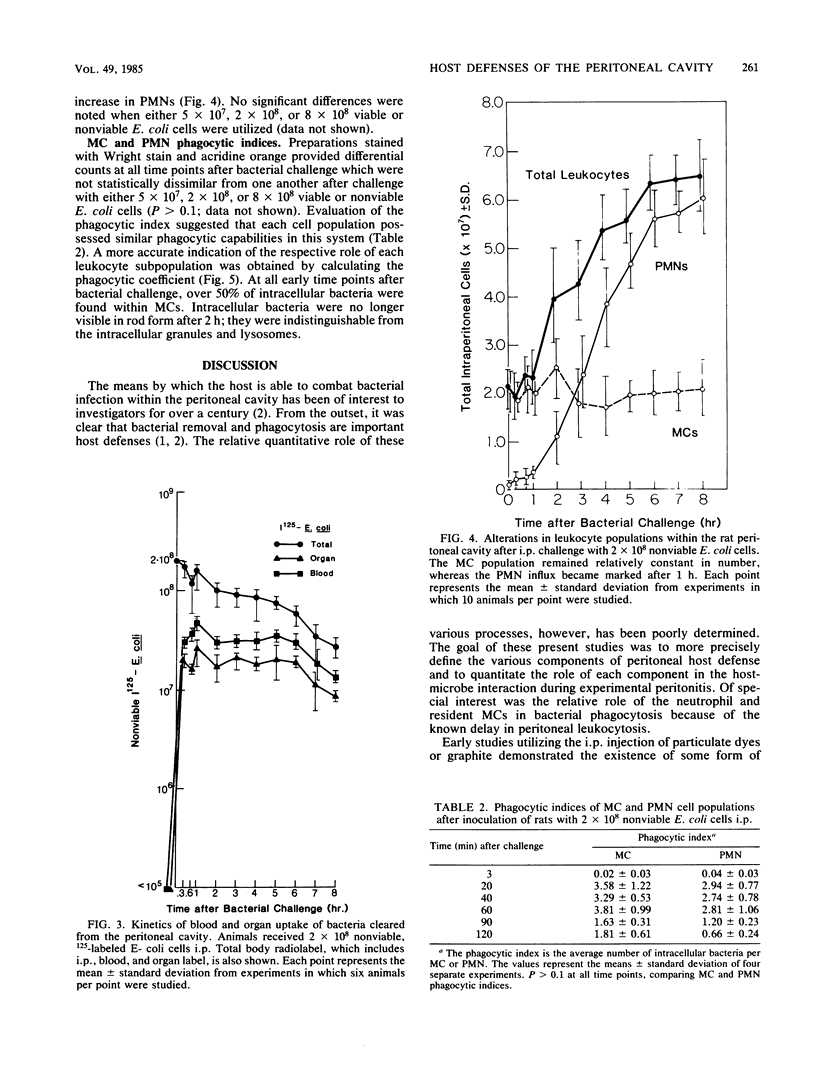
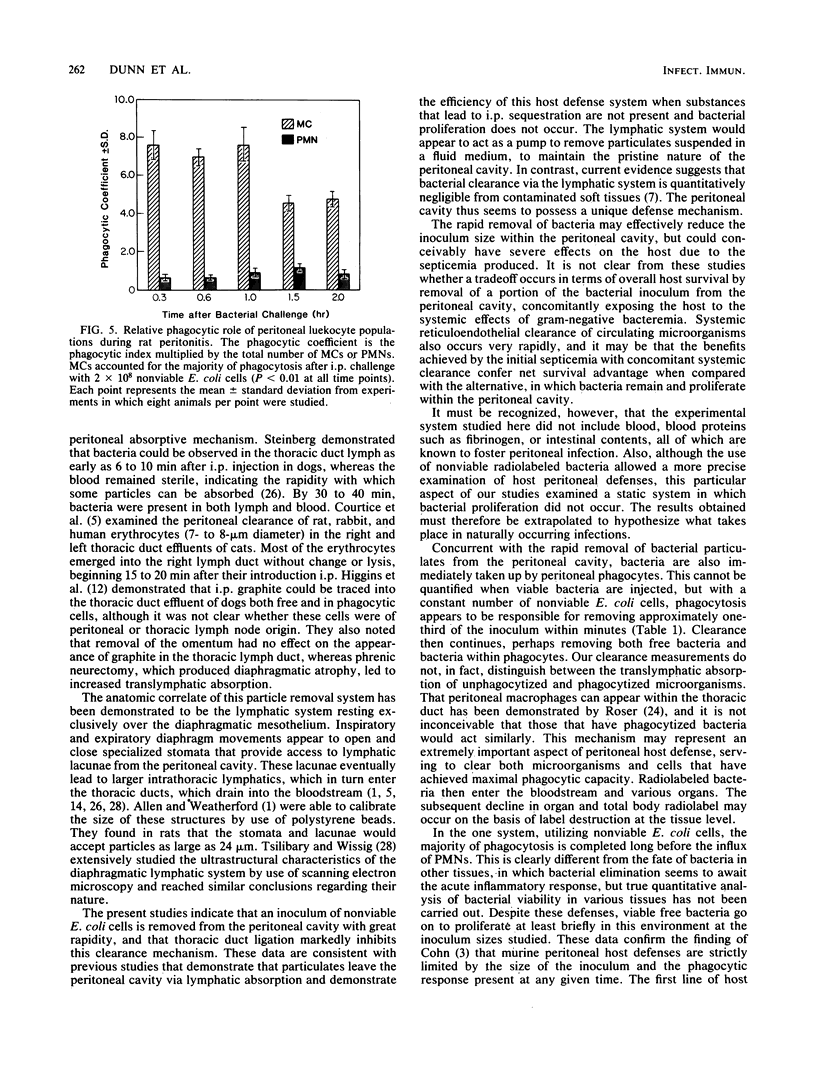
Microbial pathogens within the peritoneal cavity are thought to encounter three categories of host defense mechanisms: (i) removal mechanisms, which occur via diaphragmatic lymphatic absorption; (ii) killing mechanisms, in which host phagocytes act as effector cells; and (iii) sequestration mechanisms due to fibrin trapping and the formation of adhesions between visceral surfaces. We sought to define and quantitate the relative role of the first two components in an experimental rat model of Escherichia coli peritonitis in which fibrinous adhesions do not form. Intraperitoneal challenge with greater than or equal to 2 X 10(8) CFU of viable E. coli led to an initial decline in bacterial numbers followed by ongoing proliferation and greater than 50% mortality. With inocula of less than or equal to 5 X 10(7) CFU, elimination of bacteria occurred after moderate initial proliferation, and no mortality ensued. Nonviable, radiolabeled E. coli organisms were utilized to examine bacterial clearance via translymphatic absorption and phagocytosis. Both processes were extremely rapid, serving to eliminate free bacteria rapidly within the peritoneal cavity. Although macrophages and polymorphonuclear leukocytes within the peritoneal cavity demonstrated similar phagocytic capacities, the predominance of macrophages at the time of the initial bacterial insult led to the conclusion that these cells, in addition to translymphatic absorption, represent the first line of host defenses, acting to eliminate bacteria in the incipient stages of infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN L., WEATHERFORD T. Role of fenestrated basement membrane in lymphatic absorption from peritoneal cavity. Am J Physiol. 1959 Sep;197:551–554. doi: 10.1152/ajplegacy.1959.197.3.551. [DOI] [PubMed] [Google Scholar]
- COHN Z. A. Determinants of infection in the peritoneal cavity. I. Response to and fate of Staphylococcus aureus and Staphylococcus albus in the mouse. Yale J Biol Med. 1962 Aug;35:12–28. [PMC free article] [PubMed] [Google Scholar]
- COURTICE F. C., HARDING J., STEINBECK A. W. The removal of free red blood cells from the peritoneal cavity of animals. Aust J Exp Biol Med Sci. 1953 Jun;31(3):215–225. doi: 10.1038/icb.1953.26. [DOI] [PubMed] [Google Scholar]
- Cooper M. D., Floyd S. A. In vitro kinetics of phagocytosis and intracellular killing of gonococci by peritoneal macrophages from mice deficient in complement component 5. Infect Immun. 1982 Apr;36(1):363–370. doi: 10.1128/iai.36.1.363-370.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daems W. T., de Bakker J. M. Do resident macrophages proliferate? Immunobiology. 1982 Apr;161(3-4):204–211. doi: 10.1016/S0171-2985(82)80075-2. [DOI] [PubMed] [Google Scholar]
- DeLong T. G., Simmons R. L. Role of lymphatic vessels in bacterial clearance from early soft-tissue infection. Arch Surg. 1982 Feb;117(2):123–128. doi: 10.1001/archsurg.1982.01380260013003. [DOI] [PubMed] [Google Scholar]
- Dunn D. L., Barke R. A., Ewald D. C., Simmons R. L. Effects of Escherichia coli and Bacteroides fragilis on peritoneal host defenses. Infect Immun. 1985 May;48(2):287–291. doi: 10.1128/iai.48.2.287-291.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn D. L., Barke R. A., Lee J. T., Jr, Condie R. M., Humphrey E. W., Simmons R. L. Mechanisms of the adjuvant effect of hemoglobin in experimental peritonitis. VII. Hemoglobin does not inhibit clearance of Escherichia coli from the peritoneal cavity. Surgery. 1983 Sep;94(3):487–493. [PubMed] [Google Scholar]
- Dunn D. L., Nelson R. D., Condie R. M., Simmons R. L. Mechanisms of the adjuvant effect of hemoglobin in experimental peritonitis. VI. Effects of stroma-free hemoglobin and red blood cell stroma on mortality and neutrophil function. Surgery. 1983 May;93(5):653–659. [PubMed] [Google Scholar]
- Dunn D. L., Simmons R. L. Fibrin in peritonitis. III. The mechanism of bacterial trapping by polymerizing fibrin. Surgery. 1982 Sep;92(3):513–519. [PubMed] [Google Scholar]
- Hau T., Ahrenholz D. H., Simmons R. L. Secondary bacterial peritonitis: the biologic basis of treatment. Curr Probl Surg. 1979 Oct;16(10):1–65. doi: 10.1016/s0011-3840(79)80011-8. [DOI] [PubMed] [Google Scholar]
- JENKIN C., BENACERRAF B. In vitro studies on the interaction between mouse peritoneal macrophages and strains of Salmonella and Escherichia coli. J Exp Med. 1960 Aug 1;112:403–417. doi: 10.1084/jem.112.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings M. M., Jennings S. A., Robson M. C., Heggers J. P. Mechanisms of host defense and quantitative comparisons of bacterial populations in experimental peritonitis. Can J Microbiol. 1980 Feb;26(2):175–178. doi: 10.1139/m80-026. [DOI] [PubMed] [Google Scholar]
- Joyce L. D., Hau T., Hoffman R., Simmons R. L., Lillehei R. C. Evaluation of the mechanism of zymosan-induced resistance to experimental peritonitis. Surgery. 1978 Jun;83(6):717–725. [PubMed] [Google Scholar]
- Lee J. T., Jr, Ahrenholz D. H., Nelson R. D., Simmons R. L. Mechanisms of the adjuvant effect of hemoglobin in experimental peritonitis. V. The significance of the coordinated iron component. Surgery. 1979 Jul;86(1):41–48. [PubMed] [Google Scholar]
- Liang-Takasaki C. J., Mäkelä P. H., Leive L. Phagocytosis of bacteria by macrophages: changing the carbohydrate of lipopolysaccharide alters interaction with complement and macrophages. J Immunol. 1982 Mar;128(3):1229–1235. [PubMed] [Google Scholar]
- MacGowan A. P., Peterson P. K., Keane W., Quie P. G. Human peritoneal macrophage phagocytic, killing, and chemiluminescent responses to opsonized Listeria monocytogenes. Infect Immun. 1983 Apr;40(1):440–443. doi: 10.1128/iai.40.1.440-443.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K., Otani T., Une T., Osada Y., Ogawa H., Azuma I. Stimulation of nonspecific resistance to infection induced by muramyl dipeptide analogs substituted in the gamma-carboxyl group and evaluation of N alpha-muramyl dipeptide-N epsilon-stearoyllysine. Infect Immun. 1983 Mar;39(3):1029–1040. doi: 10.1128/iai.39.3.1029-1040.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadimitriou J. M., van Bruggen I. Cytophotometric assessment of phagocytosis and pinocytosis on single resident and exudate murine peritoneal macrophages. J Pathol. 1982 Aug;137(4):329–334. doi: 10.1002/path.1711370407. [DOI] [PubMed] [Google Scholar]
- Peterson P. K., Verhoef J., Schmeling D., Quie P. G. Kinetics of phagocytosis and bacterial killing by human polymorphonuclear leukocytes and monocytes. J Infect Dis. 1977 Oct;136(4):502–509. doi: 10.1093/infdis/136.4.502. [DOI] [PubMed] [Google Scholar]
- Radzun H. J., Dommes M., Henselmans M., Parwaresch M. R. Resident human peritoneal macrophages: a monocytic cell line. Acta Cytol. 1982 May-Jun;26(3):363–366. [PubMed] [Google Scholar]
- Roser B. J. The origin and significance of macrophages in thoracic duct lymph. Aust J Exp Biol Med Sci. 1976 Dec;54(6):541–550. doi: 10.1038/icb.1976.55. [DOI] [PubMed] [Google Scholar]
- Schnyder J., Baggiolini M. Role of phagocytosis in the activation of macrophages. J Exp Med. 1978 Dec 1;148(6):1449–1457. doi: 10.1084/jem.148.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T., Blumenstock E., Kanegasaki S. Phagocytic and chemiluminescent responses of mouse peritoneal macrophages to living and killed Salmonella typhimurium and other bacteria. Infect Immun. 1981 Jun;32(3):1242–1248. doi: 10.1128/iai.32.3.1242-1248.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsilibary E. C., Wissig S. L. Absorption from the peritoneal cavity: SEM study of the mesothelium covering the peritoneal surface of the muscular portion of the diaphragm. Am J Anat. 1977 May;149(1):127–133. doi: 10.1002/aja.1001490111. [DOI] [PubMed] [Google Scholar]
- Verbrugh H. A., Hoidal J. R., Nguyen B. Y., Verhoef J., Quie P. G., Peterson P. K. Human alveolar macrophage cytophilic immunoglobulin G-mediated phagocytosis of protein A-positive staphylococci. J Clin Invest. 1982 Jan;69(1):63–74. doi: 10.1172/JCI110442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugh H. A., Keane W. F., Hoidal J. R., Freiberg M. R., Elliott G. R., Peterson P. K. Peritoneal macrophages and opsonins: antibacterial defense in patients undergoing chronic peritoneal dialysis. J Infect Dis. 1983 Jun;147(6):1018–1029. doi: 10.1093/infdis/147.6.1018. [DOI] [PubMed] [Google Scholar]
- Verhoef J., Peterson P., Kim Y., Sabath L. D., Quie P. G. Opsonic requirements for staphylococcal phagocytosis. Heterogeneity among strains. Immunology. 1977 Aug;33(2):191–197. [PMC free article] [PubMed] [Google Scholar]
- Volkman A. Disparity in origin of mononuclear phagocyte populations. J Reticuloendothel Soc. 1976 Apr;19(4):249–268. [PubMed] [Google Scholar]
- Williams D. L., Browder I. W., Di Luzio N. R. Immunotherapeutic modification of Escherichia coli--induced experimental peritonitis and bacteremia by glucan. Surgery. 1983 Mar;93(3):448–454. [PubMed] [Google Scholar]
- van Furth R. Current view on the mononuclear phagocyte system. Immunobiology. 1982 Apr;161(3-4):178–185. doi: 10.1016/S0171-2985(82)80072-7. [DOI] [PubMed] [Google Scholar]



