Abstract
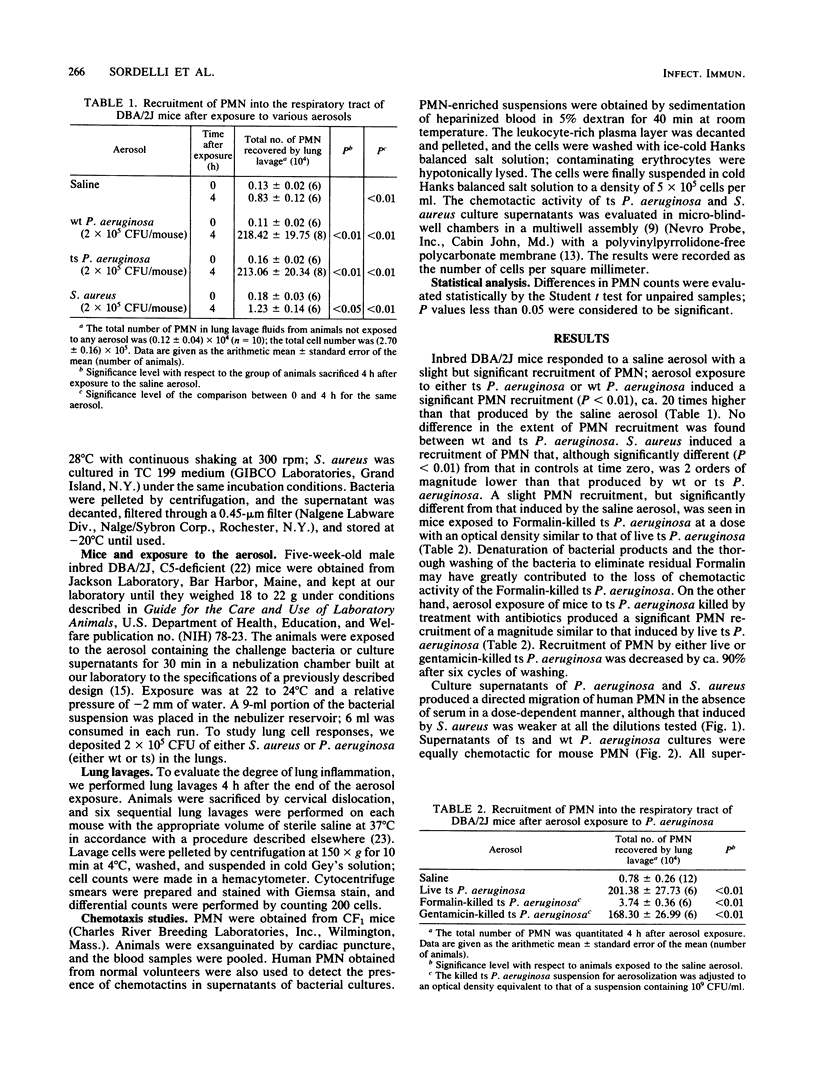
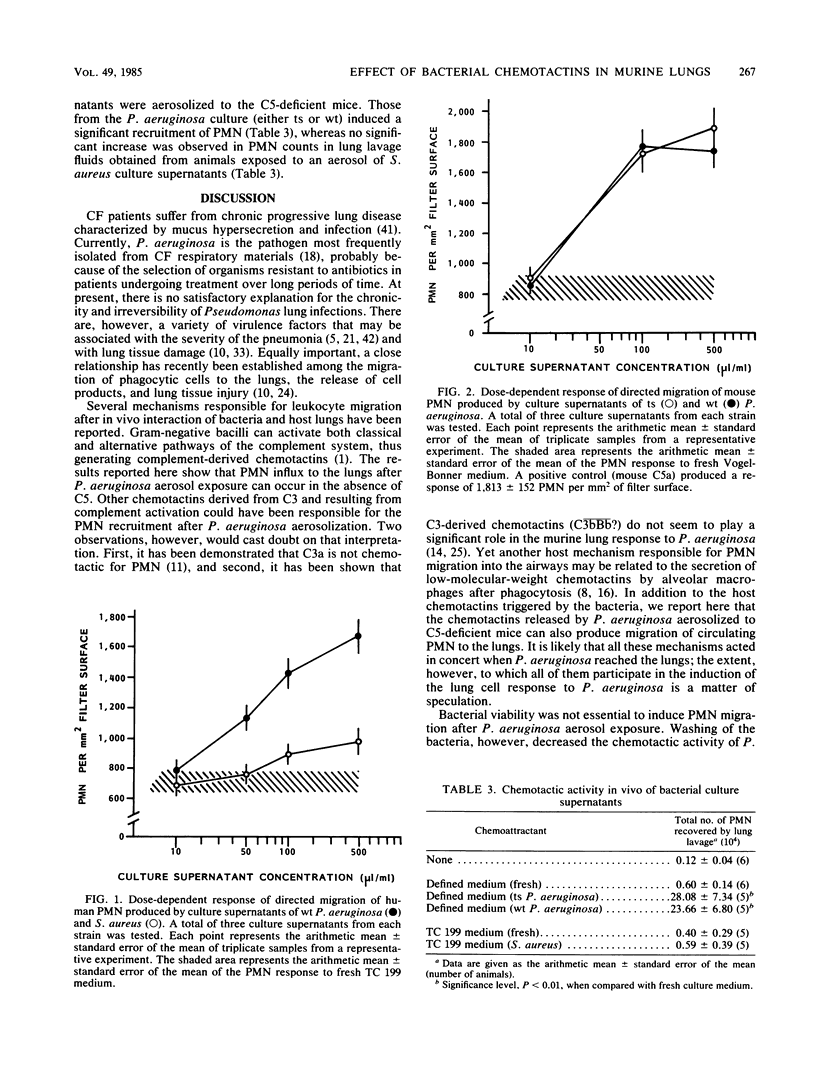
Staphylococcus aureus, the Pseudomonas aeruginosa temperature-sensitive (ts) mutant A/10/25, and the P. aeruginosa parental wild type were aerosolized to C5-deficient mice, and the total number of polymorphonuclear leukocytes (PMN) recovered by lung lavage was determined 4 h after aerosol exposure. S. aureus induced a slight but significant recruitment of PMN, as compared with the effect of a saline aerosol. Both wild-type P. aeruginosa and the ts mutant induced a significant PMN recruitment of a magnitude ca. 180 times higher than that produced by S. aureus. Gentamicin-killed ts P. aeruginosa induced a PMN recruitment of a magnitude similar to that produced by live ts P. aeruginosa. Thorough washing of the bacteria, however, removed ca. 90% of the chemotactic activity. Exposure of the animals to a ts P. aeruginosa culture supernatant aerosol induced significant PMN recruitment into the lower airways. The same culture supernatants were chemotactic for mouse PMN in a dose-dependent fashion when tested in vitro in the absence of serum. Culture supernatants of S. aureus exhibited weak chemotactic activity in vitro and did not induce PMN recruitment in the lungs when aerosolized to DBA/2J mice. The results suggest that chemotactins released by P. aeruginosa may be an important virulence factor and play a significant role in lung tissue damage.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayars G. H., Altman L. C., Rosen H., Doyle T. The injurious effect of neutrophils on pneumocytes in vitro. Am Rev Respir Dis. 1984 Jun;129(6):964–973. doi: 10.1164/arrd.1984.129.6.964. [DOI] [PubMed] [Google Scholar]
- Brain J. D. Pulmonary macrophages: when do they prevent and when do they cause COPD? Chest. 1980 Feb;77(2 Suppl):264–265. doi: 10.1378/chest.77.2.264. [DOI] [PubMed] [Google Scholar]
- Brigham K. L., Meyrick B. Granulocyte-dependent injury of pulmonary endothelium: a case of miscommunication? Tissue Cell. 1984;16(2):137–155. doi: 10.1016/0040-8166(84)90039-9. [DOI] [PubMed] [Google Scholar]
- Cash H. A., Straus D. C., Bass J. A. Pseudomonas aeruginosa exoproducts as pulmonary virulence factors. Can J Microbiol. 1983 Apr;29(4):448–456. doi: 10.1139/m83-072. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Bitterman P. B., Rennard S. I., Hance A. J., Keogh B. A. Interstitial lung diseases of unknown cause. Disorders characterized by chronic inflammation of the lower respiratory tract. N Engl J Med. 1984 Jan 26;310(4):235–244. doi: 10.1056/NEJM198401263100406. [DOI] [PubMed] [Google Scholar]
- Densen P., Mandell G. L. Phagocyte strategy vs. microbial tactics. Rev Infect Dis. 1980 Sep-Oct;2(5):817–838. doi: 10.1093/clinids/2.5.817. [DOI] [PubMed] [Google Scholar]
- Doig M. V., Ford-Hutchinson A. W. The production and characterisation of products of the lipoxygenase enzyme system released by rat peritoneal macrophages. Prostaglandins. 1980 Dec;20(6):1007–1019. doi: 10.1016/0090-6980(80)90055-6. [DOI] [PubMed] [Google Scholar]
- Falk W., Goodwin R. H., Jr, Leonard E. J. A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods. 1980;33(3):239–247. doi: 10.1016/0022-1759(80)90211-2. [DOI] [PubMed] [Google Scholar]
- Fernandez H. N., Henson P. M., Otani A., Hugli T. E. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978 Jan;120(1):109–115. [PubMed] [Google Scholar]
- HENDERSON D. W. An apparatus for the study of airborne infection. J Hyg (Lond) 1952 Mar;50(1):53–68. doi: 10.1017/s0022172400019422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada R. N., Vatter A. E., Repine J. E. Macrophage effector function in pulmonary oxygen toxicity: hyperoxia damages and stimulates alveolar macrophages to make and release chemotaxins for polymorphonuclear leukocytes. J Leukoc Biol. 1984 Apr;35(4):373–383. doi: 10.1002/jlb.35.4.373. [DOI] [PubMed] [Google Scholar]
- Harvath L., Falk W., Leonard E. J. Rapid quantitation of neutrophil chemotaxis: use of a polyvinylpyrrolidone-free polycarbonate membrane in a multiwell assembly. J Immunol Methods. 1980;37(1):39–45. doi: 10.1016/0022-1759(80)90179-9. [DOI] [PubMed] [Google Scholar]
- Heidbrink P. J., Toews G. B., Gross G. N., Pierce A. K. Mechanisms of complement-mediated clearance of bacteria from the murine lung. Am Rev Respir Dis. 1982 May;125(5):517–520. doi: 10.1164/arrd.1982.125.5.517. [DOI] [PubMed] [Google Scholar]
- Hooke A. M., Arroyo P. J., Oeschger M. P., Bellanti J. A. Temperature-sensitive mutants of Pseudomonas aeruginosa: isolation and preliminary immunological evaluation. Infect Immun. 1982 Oct;38(1):136–140. doi: 10.1128/iai.38.1.136-140.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Gallin J. I., Fauci A. S. Immunologic reactivity of the lung: the in vivo and in vitro generation of a neutrophil chemotactic factor by alveolar macrophages. Am Rev Respir Dis. 1978 Jan;117(1):15–23. doi: 10.1164/arrd.1978.117.1.15. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Garrett K. C., Richerson H. B., Fantone J. C., Ward P. A., Rennard S. I., Bitterman P. B., Crystal R. G. Pathogenesis of the granulomatous lung diseases. Am Rev Respir Dis. 1984 Sep;130(3):476–496. doi: 10.1164/arrd.1984.130.3.476. [DOI] [PubMed] [Google Scholar]
- Janoff A. Proteases and lung injury. A state-of-the-art minireview. Chest. 1983 May;83(5 Suppl):54S–58S. [PubMed] [Google Scholar]
- Kulczycki L. L., Murphy T. M., Bellanti J. A. Pseudomonas colonization in cystic fibrosis. A study of 160 patients. JAMA. 1978 Jul 7;240(1):30–34. [PubMed] [Google Scholar]
- McCord J. M. Oxygen radicals and lung injury. The state of the art. Chest. 1983 May;83(5 Suppl):35S–37S. doi: 10.1378/chest.83.5_supplement.35s-a. [DOI] [PubMed] [Google Scholar]
- Nicas T. I., Iglewski B. H. Isolation and characterization of transposon-induced mutants of Pseudomonas aeruginosa deficient in production of exoenzyme S. Infect Immun. 1984 Aug;45(2):470–474. doi: 10.1128/iai.45.2.470-474.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi Y. M., Colten H. R. Genetic defect in secretion of complement C5 in mice. Nature. 1979 Nov 8;282(5735):207–208. doi: 10.1038/282207a0. [DOI] [PubMed] [Google Scholar]
- Pivetta O. H., Cassino R. J., Sordelli D. O. Pulmonary cell response to bacterial challenge in mutant mice with some hereditary alterations resembling cystic fibrosis. Life Sci. 1980 Apr 21;26(16):1349–1357. doi: 10.1016/0024-3205(80)90096-x. [DOI] [PubMed] [Google Scholar]
- Ruddy S., Austen K. F., Goetzl E. J. Chemotactic activity derived from interaction of factors D and B of the properdin pathway with cobra venom factor or C3B. J Clin Invest. 1975 Mar;55(3):587–592. doi: 10.1172/JCI107966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. J., Wilkinson P. C., McInroy R. J., McKay S., McCartney A. C., Arbuthnott J. P. Effects of staphylococcal products on locomotion and chemotaxis of human blood neutrophils and monocytes. J Med Microbiol. 1976 Nov;9(4):433–439. doi: 10.1099/00222615-9-4-433. [DOI] [PubMed] [Google Scholar]
- Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983 May 6;220(4597):568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- Schiffmann E., Showell H. V., Corcoran B. A., Ward P. A., Smith E., Becker E. L. The isolation and partial characterization of neutrophil chemotactic factors from Escherichia coli. J Immunol. 1975 Jun;114(6):1831–1837. [PubMed] [Google Scholar]
- Schiøtz P. O., Jørgensen M., Flensborg E. W., Faerø O., Husby S., Høiby N., Jacobsen S. V., Nielsen H., Svehag S. E. Chronic Pseudomonas aeruginosa lung infection in cystic fibrosis. A longitudinal study of immune complex activity and inflammatory response in sputum sol-phase of cystic fibrosis patients with chronic Pseudomonas aeruginosa lung infections: influence of local steroid treatment. Acta Paediatr Scand. 1983 Mar;72(2):283–287. doi: 10.1111/j.1651-2227.1983.tb09712.x. [DOI] [PubMed] [Google Scholar]
- Sordelli D. O., Cerquetti M. C., Hooke A. M., Bellanti J. A. Enhancement of Pseudomonas aeruginosa lung clearance after local immunization with a temperature-sensitive mutant. Infect Immun. 1983 Mar;39(3):1275–1279. doi: 10.1128/iai.39.3.1275-1279.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordelli D. O., Zeligs B. J., Cerquetti M. C., Morris Hooke A., Bellanti J. A. Inflammatory responses to Pseudomonas aeruginosa and Staphylococcus aureus in the murine lung. Eur J Respir Dis. 1985 Jan;66(1):31–39. [PubMed] [Google Scholar]
- Suter S., Schaad U. B., Roux L., Nydegger U. E., Waldvogel F. A. Granulocyte neutral proteases and Pseudomonas elastase as possible causes of airway damage in patients with cystic fibrosis. J Infect Dis. 1984 Apr;149(4):523–531. doi: 10.1093/infdis/149.4.523. [DOI] [PubMed] [Google Scholar]
- Tainer J. A., Turner S. R., Lynn W. S. New aspects of chemotaxis. Specific target-cell attraction by lipid and lipoprotein fractions of Escherichia coli chemotactic factor. Am J Pathol. 1975 Nov;81(2):401–410. [PMC free article] [PubMed] [Google Scholar]
- Tate R. M., Repine J. E. Neutrophils and the adult respiratory distress syndrome. Am Rev Respir Dis. 1983 Sep;128(3):552–559. doi: 10.1164/arrd.1983.128.3.552. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Walker W. S., Barlet R. L., Kurtz H. M. Isolation and partial characterization of a staphylococcal leukocyte cytotaxin. J Bacteriol. 1969 Mar;97(3):1005–1008. doi: 10.1128/jb.97.3.1005-1008.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Lepow I. H., Newman L. J. Bacterial factors chemotactic for polymorphonuclear leukocytes. Am J Pathol. 1968 Apr;52(4):725–736. [PMC free article] [PubMed] [Google Scholar]
- Wilkinson P. C. Leukocyte locomotion and chemotaxis: effects of bacteria and viruses. Rev Infect Dis. 1980 Mar-Apr;2(2):293–318. doi: 10.1093/clinids/2.2.293. [DOI] [PubMed] [Google Scholar]
- Wood R. E., Boat T. F., Doershuk C. F. Cystic fibrosis. Am Rev Respir Dis. 1976 Jun;113(6):833–878. doi: 10.1164/arrd.1976.113.6.833. [DOI] [PubMed] [Google Scholar]
- Woods D. E., Cryz S. J., Friedman R. L., Iglewski B. H. Contribution of toxin A and elastase to virulence of Pseudomonas aeruginosa in chronic lung infections of rats. Infect Immun. 1982 Jun;36(3):1223–1228. doi: 10.1128/iai.36.3.1223-1228.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]



