Abstract
Overproduction of plasma cell-derived monoclonal free κ or λ immunoglobulin light chains (FLCs) is a characteristic hallmark of multiple myeloma, AL amyloidosis, and light chain deposition disease. Since these components serve as unique cellular and serologic biomarkers, their detection and quantitation has diagnostic, therapeutic, and prognostic import. In this regard, we have developed monoclonal antibodies (mAbs) that specifically recognize the κ or λ FLC products of all known human variable and constant region light-chain genes. We now report the results of our studies that have demonstrated the capability of these reagents to measure, in a modified fluid-phase capture ELISA, serum κ and λ FLCs at concentrations as low as 5 and 15 ng/mL, respectively. The mAb–based ELISA has greater sensitivity and reproducibility then does the commercially available immunoturbidimetric assay which utilizes polyclonal anti-FLC antibodies. Additionally, the mAbs can immunostain monoclonal FLC-producing plasma cells, as well as pathologic light chain-related amyloid and non-fibrillar tissue deposits. Our anti-FLC mAbs, with their high degree of reactivity and versatility, may provide an invaluable tool in the diagnosis and management of patients with light chain-associated disease.
Keywords: Free light chains, Immunoglobulins, Immunoassay, Multiple Myeloma, Amyloidosis
Monoclonal free κ and λ immunoglobulin light chains (FLCs), i.e., LCs that are not covalently bound to heavy chains (as in the case of complete IgG, IgA, IgM, IgD, and IgE molecules), have long been considered a unique biomarker of the human plasma cell–related immunoproliferative disorders which include multiple myeloma, light chain deposition disease, and AL amyloidosis.1 The deposition of these components as renal tubular casts, peritubular deposits, or amyloid fibrils leads to organ dysfunction, resulting in considerable morbidity and, eventually, death.2 Thus, the ability to measure accurately serum or urinary FLC concentrations in these patients, as well as identify by immunochemical means the clonal nature of bone marrow derived plasma cell populations and pathologic tissue deposits, has diagnostic, therapeutic, and prognostic import.
Quantification of FLCs in body fluids is necessarily dependent on the availability of antibodies that can reliably distinguish between such components and those bound to heavy chains. In this regard, efforts have been directed to developing anti-FLC mAbs that would prove suitable as diagnostic RIA- or ELISA-based reagents.3–6 Unfortunately, in most instances, these were of limited value due to lack of specificity, e.g., their cross-reactivity with Ig heavy chains or inability to recognized λ as well as κ FLCs. Furthermore, analyses of normal human sera yielded widely disparate results.7 Nonetheless, as part of our long standing effort to generate mAbs that recognize human κ or λ LC variable region (VL) subgroups or gene families (Vκ or Vλ, respectively), as well as epitopes shared among all κ or λ proteins, we identified two antibodies – one designated Fκ-C8 and the other Fλ-G9 – that reacted exclusively with κ or λ FLCs, respectively, and which could be used in a fluid-phase ELISA to measure the concentration of such molecules in blood, urine, or cerebrospinal fluid with a high degree of sensitivity and reproducibility.8,9
We now have optimized this assay to include (as the first step) capture of the murine anti-FLC mAbs in wells coated with a goat anti-mouse IgG (Fc-specific) antibody, in lieu of binding the mAbs directly to the wells. This modification, as we report, has resulted in increased accuracy and reproducibility, with the ability to measure κ and λ FLCs at concentrations as low as ~5 and 15 ng/mL, respectively. The diagnostic versatility of these reagents also has been evidenced immunocytologically and immunohistochemically, where we showed that they could be used to detect FLC-containing clonal plasma cell populations, as well as establish the light chain nature of pathologic tissue deposits, respectively.
Materials and Methods
Clinical samples
Serum, urine, and bone marrow specimens were derived from 71 patients with monoclonal plasma B-cell-related disorders (multiple myeloma, light chain deposition disease, AL amyloidosis, Waldenström's macroglobulinemia). Additionally, sera were obtained from 41 healthy adults. All blood-derived samples were analyzed within 24 hours or frozen at −20°C until use. The study was approved by the University of Tennessee Graduate School of Medicine's Institutional Review Board.
Proteins
κ- and λ- type Bence Jones proteins were isolated from the urine of patients with multiple myeloma by zone electrophoresis and their purity confirmed by SDS/PAGE, as described previously.10 The LC variable region (VL) subgroup (Vκ1, Vκ2, Vκ3, Vκ4/Vλ1, Vλ2, Vλ3, Vλ6, Vλ8) and constant region (CL) allotype (κm1,−2, κm1,2, κm3) or isotype (Cλ1, Cλ2/3, Cλ7) were established serologically and confirmed by amino acid sequence analyses or tandem mass spectrometry.11,12 Polyclonal FLCs were isolated by gel filtration chromatography of reduced-alkylated IgG (Fr II γ-globulin, Sigma, St. Louis, MO), as described.13 Polyclonal IgG, IgA, and IgM preparations were purchased from Sigma. Protein concentrations were measured in a bicinchonic acid colorimetric assay (micro BCA assay; Pierce Biotechnology, Inc., Rockford, IL).
FLC Immunoassays
Details regarding the derivation, purification, and characterization of the IgG1 anti-κ(Fκ-C8) and anti-λ (Fλ-G9) FLC mAbs have been reported previously.8,9 Briefly, Balb/c mice were immunized with thermally-denatured κ or λ Bence Jones proteins and FLC-specific IgG antibodies generated from hybridomas comprised of murine immune splenocytes fused with SP 2/0 cells, which then were subcloned, propagated, and injected i.p. into pristane-primed mice. The resultant mAbs were purified from ascitic fluid by 40% ammonium sulfate precipitation followed by ion exchange chromatography.
The FLC immunoassay was modified from that originally described9 as follows: Wells in high-titer, polystyrene microtiter plates (Costar, Corning, NY) were coated overnight at 4°C with goat anti-mouse IgG Fc-specific antibody (Sigma) diluted to a concentration of 4 µg/mL in 0.01 M sodium bicarbonate buffer, pH 9.6. The wells were washed with 0.05% Tween–20 in phosphate-buffered saline (PBST) and blocked with 1% bovine serum albumin (BSA) in PBS for 1 hour at room temperature, followed by addition of 50 µL of murine anti-FLC mAb Fκ-C8 or Fλ-G9 that was diluted in working buffer (0.1% BSA/PBS/0.01% thimerasol) to 4 µg/mL. After incubation (1 hour at 22°C), excess antibody was removed by washing ×3 with PBST. Next, wells were filled with 50-µL volumes of standards comprised of equimolar amounts of Bence Jones proteins representing the 4 Vκ or 5 Vλ subgroups diluted in working buffer in the range of 0.001 to 1 µg/mL, or individual serum samples (centrifuged at 10,000 rpm for 5 minutes at 4°C to remove any particulates) diluted 1:10, 1:30, 1:100, 1:1000, 1:3000, and 1:30,000. After a 1-hour incubation at 22°C and 3 washes with PBST, the wells were filled with 50 µL of HRP-conjugated goat F(ab′)2 anti-human κ or λ antibodies (Biosource, Camarillo, CA) diluted 1:1000, the plates incubated for 1 hour at room temperature, and excess antibody removed by washing ×3 with PBST. For color development, 50 µL of the 2-component ABTS Peroxidase Substrate System (KPL, Gaithersburg, MD) were added to each well and the enzymatic reaction terminated after 10 minutes by addition of an equal volume of 2% oxalic acid. Absorbance was measured at 405 nm using a microplate reader (BioTek Synergy HT, Winooski, VT). All assays were done in triplicate and considered valid when the R2 values of the standard curves were greater than 0.99.
For the competition ELISA, after capture of the anti-FLC mAbs, wells were filled with 50 µL of a biotinylated κ or λ Bence Jones protein (1 µg/mL) mixed with an equal volume of the same unbiotinylated Bence Jones protein or polyclonal Igs (0.1–10 µg/mL) and the plates were shaken for 1 hour at 22°C. After washing ×3 with PBST, 50 µL of Immunopure streptavidin-HRP (250 ng/mL) (Pierce Biotechnology, Inc., Rockford, IL)) were added and the plates shaken for 1 hour at room temperature. The wells were washed ×3 with PBST and then filled first with the ABTS substrate and next the oxalic acid solution, after which the absorbance was measured as described above.
For comparative purposes, serum FLC concentrations were measured using the FREELITE™ Human Kappa and Lambda Free Kits purchased from the Binding Site (San Diego, CA) and the Beckman Coulter IMMAGE™ system (Fullerton, CA), according to the manufacturers’ protocols.
Immunochemistry
For immunophenotypic analyses, bone marrow plasma cells were isolated and immunostained with the anti-κ and -λ FLC mAbs, as well as with a mouse anti-human plasma cell mAb (Clone VS38c, Dako, Carpinteria, CA), as described previously.14 Immunohistochemical studies were performed using 4 µm-thick formalin-fixed, paraffin-embedded tissue sections mounted on poly-L-lysine-coated slides that were dried overnight at room temperature and deparaffinized. Sections were immersed in the Glyca pH 4.0 antigen-retrieval solution, heated in a microwave oven, and then immunostained using the ImmPRESS polymerized reporter enzyme-linked system (Vector Laboratories, Burlingame, CA).
RESULTS
Specificity of Anti-Human κ and λ FLC mAbs
The anti-κ (Fκ-C8) and -λ (Fλ-G9) FLC mAbs were shown in a competitive inhibition ELISA to react specifically with monoclonal or polyclonal FLCs, but not with these molecules when they were covalently bound to γ, α, or µ heavy chains (i.e., IgG, IgA, or IgM proteins). Table 1. As shown in Figure 1, κ and λ Bence Jones proteins effectively inhibited the interaction of mAbs Fκ-C8 and Fλ-G9, respectively, with isotype-matched counterparts coated on ELISA plate wells.
Table 1.
Reactivity of Anti-FLC mAbs with Monoclonal and Polyclonal Human Immunoglobulins (Igs) and Light Chains (LCs)*
| Proteins | anti-κ FLC (Fκ-C8) | anti-λ FLC (Fλ-G9) |
|---|---|---|
| Monoclonal Igs | ||
| IgG | 0 | 0 |
| IgA | 0 | 0 |
| IgD | 0 | 0 |
| IgM | 0 | 0 |
| Polyclonal Igs | ||
| IgG | 0 | 0 |
| IgA | 0 | 0 |
| IgM | 0 | 0 |
| Monoclonal LCs | ||
| κ | + | 0 |
| λ | 0 | + |
| Polyclonal LCs | + | + |
0, no difference from background; +, >0.45 O.D.
Figure 1.
Anti-FLC specificity of mAbs Fκ-C8 and Fλ-G9. Competitive ELISA in which 1 µg/mL of a biotinylated κ or λ Bence Jones protein was incubated with 0.1–10 µg/mL of unbiotinylated κ (■) or λ (●) Bence Jones protein, as well as with polyclonal IgG (◆), IgA (▼), or IgM (▲).
Additional experimental data confirmed that the epitope recognized by the anti-FLC mAbs was confined to the CL domain, as evidenced by the fact that mAbs Fκ-C8 and Fλ-G9 had comparable reactivity with proteins representative of the major human Vκ and Vλ subgroups (Vκ1, Vκ2, Vκ3, Vκ4 and Vλ1, Vλ2, Vλ3, Vλ6, Vλ8), respectively. Furthermore, the CL specificity of these reagents was found to be independent of the Cκ allotype (κm1,−2, κm1,2, κm3) or Cλ isotype (Cλ1, Cλ2/3, Cλ7) Table 2. Additionally, the presence of carbohydrate on Bence Jones proteins (as demonstrated in PAS-stained SDS/PAGE gels) did not affect the assay; similar results were obtained in analyses of monomers and dimers isolated from individual samples by gel filtration chromatography. In contrast, reduction and alkylation of monoclonal LCs in the presence of 6 M guanidine HCl or enzymatic cleavage of such molecules into VL and CL fragments15 resulted in loss of reactivity with the anti-FLC mAbs (data not shown).
Table 2.
Reactivity of Anti-FLC mAbs with Bence Jones Proteins Representative of the Human Vκ/Vλ Gene Families, Cκ Allotypes, and Cλ Isotypes*
| VL/CL | anti-κ FLC mAb (Fκ-C8) | anti-λ FLC mAb (Fλ-G9) |
|---|---|---|
| Vκ1 | + | 0 |
| Vκ2 | + | 0 |
| Vκ3 | + | 0 |
| Vκ4 | + | 0 |
| κm1,−2 | + | 0 |
| κm1,2 | + | 0 |
| κm3 | + | 0 |
| Vλ1 | 0 | + |
| Vλ2 | 0 | + |
| Vλ3 | 0 | + |
| Vλ6 | 0 | + |
| Vλ8 | 0 | + |
| Cλ1 | 0 | + |
| Cλ2/3 | 0 | + |
| Cλ7 | 0 | + |
0, no difference from background; +, >0.45 O.D.
FLC Immunoassay
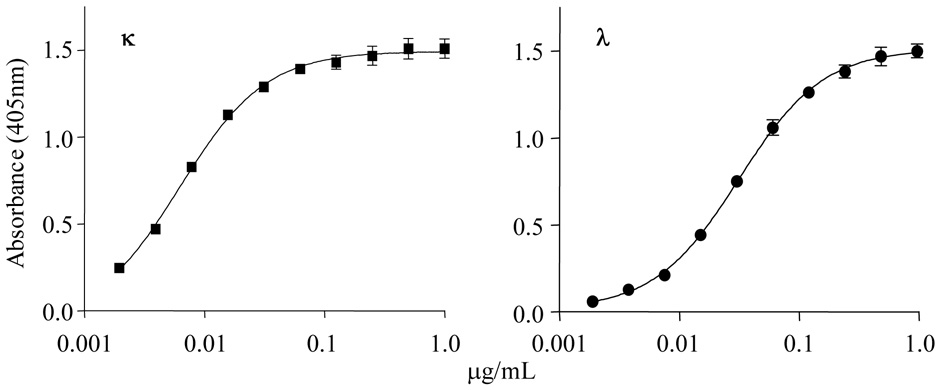
Initially, standard calibration curves for κ or λ FLCs were generated using equimolar pools of κ1, κ2, κ3, κ4 or λ1, λ2, λ3, λ6, λ8 Bence Jones proteins, respectively, that were tested in triplicate against the anti-FLC mAbs over a 4-log concentration (0.001 to 1 µg/mL). As indicated in Figure 2, the resultant data were linear to a minimum/maximum for κ of 0.005/0.15 and, for λ, 0.015/0.065 µg/mL, and were reproducible, as evidenced by the small S.D. values. Since comparable results were obtained using a single Bence Jones protein representative of each isotype, subsequent studies were done with one such component serving as the standard in each assay.
Figure 2.
Standard κ and λ FLC curves. Pools of κ (■) and λ (●) Bence Jones proteins representative of the major human Vκ and Vλ subgroups were serially diluted and subjected to the anti-FLC capture immunoassay. The results (n=6) are plotted (bars indicate the S.D.).
To establish a reference range for κ and λ FLCs in the blood of normal individuals, specimens from 19 healthy males and 22 females from ages 29 to 67 years were tested where, for κ, a range of 4.2 – 13.0 mg/L was found and for λ, 16.4 – 127.3 mg/L, giving κ:λ ratios between 0.07–0.28. The mean values ± S.D. for each sex and the combined group are given in Table 3. Although statistically significant quantitative differences were seen in the κ and λ values obtained in males versus females (p=0.003 and 0.015, respectively), the κ:λ FLC ratios were comparable and not age dependent. Further, tests of serum versus plasma yielded equivalent results. The intra-assay coefficients of variation (40 replicates) for κ and λ were 5.6 and 4.3% and the inter-assay coefficients of variation (10 plates) were 7.1 and 8.1%, respectively. Analyses of pre-treatment specimens from 71 patients with the LC-associated disorders multiple myeloma (36), systemic AL amyloidosis (23), and light chain deposition disease (7), as well as Waldenström's macroglobulinemia (5), revealed abnormal κ:λ FLC ratios of >0.3 in cases having monoclonal κ-type serum Igs and/or urinary Bence Jones proteins (as evidenced by immunofixation electrophoresis) or, alternatively, <0.07 for those with λ-type molecules. In all instances, there was concordance between the κ or λ nature of the serum or urinary monoclonal protein and its predominance in the FLC ratio. In general, a value that was abnormally high or low resulted from an increase in the amount of the monoclonal Ig; however, the extent of deviation of FLC values from normal was not necessarily related to the concentration of the pathologic component. This contrasted with the unaffected isotype which was within normal limits or reduced. In patients who responded to therapy, a reduction in the concentration of the monoclonal serum or urinary Ig was reflected by normalization of the κ:λ FLC ratio, though even when such molecules could no longer be detected post-therapy by immunofixation electrophoresis, a normal FLC ratio was not necessarily found.
Table 3.
ELISA mAb-Derived FLC values (mg/L) in Normal Adult Sera (mean ± S.D.)
| κ FLC | λ FLC | κ:λ FLC* | |
|---|---|---|---|
| Males (19) | 6.6 ± 2.3 | 49.8 ± 21.5 | 0.15 ± 0.06 |
| Females (22) | 8.9 ± 2.8 | 66.1 ± 27.5 | 0.14 ± 0.04 |
| Combined (41) | 7.9 ± 2.8 | 58.5 ± 26.0 | 0.15 ± 0.05 |
Average of all individual patient values.
Comparison of mAb and Polyclonal Antibody-Based FLC Immunoassays
The results of our analyses of serum specimens using our mAb-based ELISA methodology versus the FREELITE™ immunoturbidimetric method that utilizes polyclonal reagents revealed marked quantitative differences in the κ and λ values; in the case of FREELITE™, these were generally lower (especially for λ) than those obtained by ELISA, thus affecting the absolute FLC κ:λ ratios. As shown in Table 4, these trends also were apparent when the same 12 serum specimens were analyzed in our laboratory and in that of Dr. Jerry A. Katzman at the Mayo Clinic, where the most notable discrepancy involved Patient 3 who had an IgGλ gammopathy. Further, in our studies of 71 monoclonal Ig-containing sera, κ and λ data could be obtained directly in all samples by the ELISA method, whereas this was only possible in 39 instances using the standard 1:10 dilution specified by the FREELITE™ protocol (in the other 32 cases, it was necessary to repeat the study using, as appropriate, undiluted or offline diluted specimens).
Table 4.
Comparison of FLC Immunoquantitative Data
| Patient/Diagnosis* | Monoclonal Ig † | anti-FLC polyclonal antibodies‡ | anti-FLC monoclonal antibodies§ | ||||
|---|---|---|---|---|---|---|---|
| κ FLC∥¶ | λ FLC∥¶ | κ:λ FLC¶ | κ FLC∥# | λ FLC∥# | κ:λ # | ||
| FLC¶ | |||||||
| 1/AL | IgGλ | 1.1 | 10.9 | 0.10 | 0.6 | 27.7 | 0.02 |
| 2/MM | IgGκ | 4.1 | 0.6 | 6.83 | 6.6 | 6.2 | 1.06 |
| 3/AL | IgGλ | 1.2 | 1.9 | 0.63 | 1.3 | 50.5 | 0.03 |
| 4/AL | IgGκ | 12.1 | 1.6 | 7.56 | 18.1 | 19.9 | 0.91 |
| 5/AL | BJPκ | 101.0 | 0.2 | 505.0 | 141.5 | 4.1 | 34.51 |
| 6/AL | IgGλ/BJPλ | 0.8 | 40.0 | 0.02 | 1.3 | 43.3 | 0.03 |
| 7/MM | IgGκ | 68.1 | 0.26 | 261.9 | 27.2 | 2.8 | 9.71 |
| 8/MM/AL | BJPκ | 39.1 | 4.20 | 9.31 | 282.1 | 25.6 | 11.02 |
| 9/MM | BJPλ | 0.15 | 74.7 | 0.002 | 0.7 | 71.4 | 0.01 |
| 10/AL | IgAλ | 0.62 | 8.1 | 0.08 | 0.7 | 217.3 | 0.003 |
| 11/AL | BJPλ | 0.96 | 48.7 | 0.02 | 1.2 | 27.8 | 0.04 |
| 12/MM | BJPκ | 35.8 | 1.1 | 32.5 | 35.5 | 6.0 | 5.92 |
AL, AL amyloidosis; MM, multiple myeloma.
Based on immunofixation electrophoresis.
FREELITE™ anti-κ FLC and anti-λ FLC reagents.
anti-κ FLC (Fκ–C8) and anti-λ FLC (Fλ–G9) mAbs.
mg/L.
Normal values: κ, 3.3–19.4; λ, 5.71–26.3; κ:λ, 0.26–1.65.
Normal values: κ, 4.2–13.0; λ, 16.4–127.3; κ:λ, 0.07–0.28.
Immunohistochemistry
The specific recognition of κ and λ FLCs by mAbs Fκ-C8 and Fλ-G9 also was demonstrated immunocytochemically when these reagents were tested against cell suspensions prepared from the bone marrow of patients with multiple myeloma or AL amyloidosis. Among more than 30 cases studied, the monoclonal Ig immunophenotype of the plasma cells was apparent by a predominance of κ or λ cytoplasmic reactivity Image 1, even in those patients with AL amyloidosis in whom the percentage of this cell population was relatively low, i.e., <5%. There was complete agreement between the type of FLC documented immunocytochemically and that of the serum or urinary monoclonal protein. Notably, κ or λ FLCs could be detected in plasma cells from those individuals who had monoclonal serum Igs, but no evident Bence Jones protein, even when urine specimens were tested at a concentration of ≥ 100 mg/mL.
Image 1.
Immunocytochemical detection of κ or λ FLCs in bone marrow-derived plasma cells. Cytospin suspension of preparations obtained from patients with multiple myeloma (Panels A and B) or AL amyloidosis (Panels C and D) and immunostained, using as primary reagents, an anti-plasma cell antibody or the anti-κ and -λ FLC mAbs Fκ-C8 and Fλ-G9, respectively (original magnifications, ×400)
The anti-FLC mAbs also immunostained AL amyloid tissue deposits Image 2, as well as other types of pathologic LC precipitates, including those found in the form of κ- or λ-containing casts in renal tubules or as peritubular punctate lesions as found in multiple myeloma or light chain deposition disease, respectively Image 3.
Image 2.
Immunohistochemical detection of κ- or λ-containing AL amyloid deposits. Formalin-fixed, deparaffinized sections of kidney (Panels A and B) and heart (Panels C and D) were stained with Congo red and examined by polarizing microscopy, as well as immunostained as described in Image 1 (original magnifications ×400).
Image 3.
Immunohistochemical detection of κ- or λ-containing pathologic deposits. Formalin–fixed, deparaffinized sections of kidney obtained from patients with multiple myeloma (Panels A and B) and light chain deposition disease (Panel C) immunostained as described in Image 1 (Original magnifications, ×400).
DISCUSSION
We have developed a robust fluid–phase capture ELISA that utilizes specific murine anti-human FLC mAbs to measure, with a high degree of sensitivity and reproducibility, concentrations of κ and λ FLCs in serum and other body fluids. Further, these mAbs have proven to be useful as immunodiagnostic reagents to establish the monoclonality of bone-marrow derived plasma cells, especially in the case of AL amyloidosis, where in contrast to multiple myeloma, the percentage is typically low, i.e., 5–10%.16 Additionally, in immunohistochemical studies, the κ or λ nature of amyloid and non-fibrillar pathologic deposits found in tissue biopsies was readily evidenced. As shown by others,17–26 measurement of FLCs in serial blood or urine specimens obtained from patients pre- and post-chemotherapy can be used to document response to treatment and/or relapse. The clinical import of such information is the subject of a recent review article.27
Although it is presumed that the FLC epitope recognized by mAbs Fκ-C8 and Fλ-G9 is located in the CL domain, its expression requires the intact LC molecule, inasmuch as it was not detected on pepsin-derived or naturally occurring CL fragments. In this respect, we previously have reported that serologic identification of κ and λ epitopes, as well as κm allotypes (located in the Cκ region), also was dependent on the native polypeptide.15,28–30 In studies involving a non-secreted aberrant κ LC product, that due to a mis-sense mutation had a completely anomalous sequence after position 187 (including the absence of the Cys 194 residue required for an intrachain disulfide bond with Cys 146), we found that this component was non-reactive with the anti-κ FLC mAb and posited that the epitope recognized by this antibody was located between residues 187 and 214, but depended upon the structural integrity of the entire LC molecule.31
Currently, the only commercially available FLC assay for clinical use is the Binding Site Inc.’s FREELITE™ system. This immunoassay is based on the interaction of affinity purified sheep anti-human κ or λ FLC polyclonal antibody coated latex particles with FLCs contained in serum or urine; the resultant turbidity is compared to reference standards and measured by nephelometry.32,33 The FREELITE™ protocol specifies that serum specimens first be diluted 1:10; however, due to its limited measuring range (particularly for λ FLCs), the manufacturer suggests that out-of-range samples be re-analyzed using a series of offline dilutions. This is seldom a problem with our ELISA-based mAb methodology since 6 different serum dilutions (1:10 to 1:30,000) are included on each plate and, generally, the results of at least 2 fall within the linear portion of the standard curve; furthermore, in contrast to the immunoturbidimetric assay, it is far more sensitive (i.e., 5 and 15 ng/mL versus 3,600 and 5,600 ng/mL32 for κ and λ FLCs, respectively) and encompasses a wider measuring range by a factor of ~1000. Notably, it is not adversely affected by lipemic or hemolyzed sera. Although it has been stated that the turbidimetric assay many be more suitable for routine immunochemistry laboratories,32 newer instruments now are available that are capable of ELISA, as well as nephelometric analyses.
We also have shown that our anti-free κ and λ mAbs react with all LCs, irrespective of their VL or CL composition. Comparison of FLC quantitative data obtained using the FREELITE™ polyclonal antibody-based immunoturbidimetric method versus our mAb ELISA-based immunoassay revealed that, with the exception of patient 3, there was agreement between the κ or λ nature of the monoclonal Ig and the abnormal κ:λ FLC ratio. However, there were notable differences in the two systems in regard to the concentrations of κ and λ FLCs (and the κ:λ ratio) in normal and patient sera. The most striking discrepancy involved the lower FREELITE™ λ values that, in turn, affected the κ:λ ratio. This may be due to the inability of the sheep anti-λ FLC antibodies to recognize all Vλ subgroups or Cλ isotypes, the confounding effect of high molecular weight λ-chain polymers,34 interference by intact Igλ molecules, instrumentation, or unknown technical factors. Other investigators have reported aberrations in measurement of FLCs by this nephelometric-based assay that result from analytic imprecision or discrepancies resulting from dilution.35,36 An additional problem can be the lot-to-lot variation and, in this regard, the use of monoclonal rather than polyclonal anti-FLC antibodies is obviously advantageous. Nonetheless, given the importance of FLC detection and measurement in the diagnosis of clonal plasma cell-related disorders, as well as for objective documentation of response to therapy and/or disease progression, it is necessary to develop, as recommended,6,7 international reference standards and validate the FLC immunoassays designed for clinical use.
Acknowledgements
We thank Craig Wooliver and James Foster for technical assistance, and Keira Clark for manuscript preparation.
Supported in part by USPHS Research Grant CA10056 from the National Cancer Institute. LXT was an American Cancer Society Harry and Elsa Jiler International Visiting Scientist and AS is an American Cancer Society Clinical Research Professor.
References
- 1.Solomon A. Monoclonal immunoglobulins as biomarkers of cancer. In: Sell S, editor. Cancer Markers: Developmental and Diagnostic Significance. Clifton, NJ: Humana Press; 1980. pp. 57–87. [Google Scholar]
- 2.Solomon A, Weiss DT, Herrera GA. Renal diseases associated with multiple myeloma and related plasma cell dyscrasias. In: Berenson JR, editor. Biology and Management of Multiple Myeloma. Totowa, NJ: Humana Press; 2004. pp. 281–302. [Google Scholar]
- 3.Ling NR, Lowe J, Hardie D, Evans S, Jefferis R. Detection of free κ chains in human serum and urine using pairs of monoclonal antibodies reacting with Cκ epitopes not available on whole immunoglobulins. Clin Exp Immunol. 1983;52:234–240. [PMC free article] [PubMed] [Google Scholar]
- 4.Axiak SM, Krishnamoorthy L, Guinan J, Raison RL. Quantitation of free kappa light chains in serum and urine using a monoclonal antibody based inhibition enzyme-linked immunoassay. J Immunol Methods. 1987;99:141–147. doi: 10.1016/0022-1759(87)90043-3. [DOI] [PubMed] [Google Scholar]
- 5.Nelson M, Brown RD, Gibson J, Joshua DE. Measurement of free kappa and lambda chains in serum and the significance of their ratio in patients with multiple myeloma. Brit J Haematol. 1992;81:223–230. doi: 10.1111/j.1365-2141.1992.tb08211.x. [DOI] [PubMed] [Google Scholar]
- 6.Nakano T, Nagata A. ELISAs for free light chains of human immunoglobulins using monoclonal antibodies: comparison of their specificity with available polyclonal antibodies. J Immunol Methods. 2003;275:9–17. doi: 10.1016/s0022-1759(02)00512-4. [DOI] [PubMed] [Google Scholar]
- 7.Nakano T, Miyazaki S, Takahashi H, et al. Immunochemical quantification of free immunoglobulin light chains from an analytical perspective. Clin Chem Lab Med. 2006;44:522–532. doi: 10.1515/CCLM.2006.118. [DOI] [PubMed] [Google Scholar]
- 8.Abe M, Goto T, Kennel SJ, et al. Production and immunodiagnostic applications of antihuman light chain monoclonal antibodies. Amer J Clin Pathol. 1993;100:67–74. doi: 10.1093/ajcp/100.1.67. [DOI] [PubMed] [Google Scholar]
- 9.Abe M, Goto T, Kosaka M, et al. Differences in kappa to lambda (κ:λ) ratios of serum and urinary free light chains. Clin Exp Immunol. 1998;111:457–462. doi: 10.1046/j.1365-2249.1998.00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solomon A. Light chains of human immunoglobulins. Methods Enzymol. 1985;116:101–121. doi: 10.1016/s0076-6879(85)16008-8. [DOI] [PubMed] [Google Scholar]
- 11.Murphy CL, Eulitz M, Hrncic R, et al. Chemical typing of amyloid protein contained in formalin-fixed paraffin-embedded biopsy specimens. Am J Clin Pathol. 2001;116:135–142. doi: 10.1309/TWBM-8L4E-VK22-FRH5. [DOI] [PubMed] [Google Scholar]
- 12.Murphy CL, Wang S, Williams T, et al. Characterization of systemic amyloid deposits by mass spectrometry. Methods Enzymol. 2006;412:48–62. doi: 10.1016/S0076-6879(06)12004-2. [DOI] [PubMed] [Google Scholar]
- 13.Fleischman JB, Pain RH, Porter RR. Reduction of gamma-globulins. Arch Biochem Biophys Suppl. 1. 1962:174–180. [PubMed] [Google Scholar]
- 14.Solomon A, Weiss DT, Macy SD, et al. Immunocytochemical detection of kappa and lambda light chain V region subgroups in human B-cell malignancies. Am J Pathol. 1990;137:855–862. [PMC free article] [PubMed] [Google Scholar]
- 15.Solomon A, McLaughlin CL. Bence-Jones proteins and light chains of immunoglobulins. I. Formations and characterization of amino-terminal (variant) and carboxyl-terminal (constant) halves. J Biol Chem. 1969;244:3393–3404. [PubMed] [Google Scholar]
- 16.Kyle RA, Gertz MA. Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Semin Hematol. 1995;8:32–59. [PubMed] [Google Scholar]
- 17.Drayson M, Tang LX, Drew R, et al. Serum free light-chain measurements for identifying and monitoring patients with nonsecretory multiple myeloma. Blood. 2001;97:2900–2902. doi: 10.1182/blood.v97.9.2900. [DOI] [PubMed] [Google Scholar]
- 18.Lachman HJ, Gallimore R, Gillmore JD, et al. Outcome in systemic AL amyloidosis in relation to changes in concentration of circulating free immunoglobulin light chains following chemotherapy. Br J Haematol. 2003;122:78–84. doi: 10.1046/j.1365-2141.2003.04433.x. [DOI] [PubMed] [Google Scholar]
- 19.Abraham RS, Katzmann JA, Clark RJ, et al. Quantitative analyses of serum free light chains. A new marker for the diagnostic evaluation of primary systemic amyloidosis. Amer J Clin Pathol. 2003;119:274–278. doi: 10.1309/LYWM-47K2-L8XY-FFB3. [DOI] [PubMed] [Google Scholar]
- 20.Rajkumar SV, Kyle RA, Therneau TM, et al. Presence of monoclonal free light chains in the serum predicts risk of progression in monoclonal gammopathy of undetermined significance. Br J Haematol. 2004;127:308–310. doi: 10.1111/j.1365-2141.2004.05169.x. [DOI] [PubMed] [Google Scholar]
- 21.Mead GP, Carr-Smith HD, Drayson MT, et al. Serum free light chains for monitoring multiple myeloma. Br J Haematol. 2004;126:348–354. doi: 10.1111/j.1365-2141.2004.05045.x. [DOI] [PubMed] [Google Scholar]
- 22.Tate J, Mollee P, Gill D. Serum free light-chains for monitoring multiple myeloma. Br J Haematol. 2005;128:405–406. doi: 10.1111/j.1365-2141.2004.05338.x. [DOI] [PubMed] [Google Scholar]
- 23.Palladini G, Lavatelli F, Russo P, et al. Circulating amyloidogenic free light chains and serum N-terminal natriuretic peptide type B decrease simultaneously in association with improvement of survival in AL. Blood. 2006;107:3854–3858. doi: 10.1182/blood-2005-11-4385. [DOI] [PubMed] [Google Scholar]
- 24.van Rhee F, Bolejack V, Hollmig K, et al. High serum-free light chain levels and their rapid reduction in response to therapy define an aggressive multiple myeloma subtype with poor prognosis. Blood. 2007;110:827–832. doi: 10.1182/blood-2007-01-067728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris KL, Tate JR, Gill D, et al. Diagnostic and prognostic utility of the serum free light chain assay in patients with AL amyloidosis. Intern Med. 2007;37:456–463. doi: 10.1111/j.1445-5994.2007.01368.x. [DOI] [PubMed] [Google Scholar]
- 26.Dispenzieri A, Kyle RA, Katzmann JA, et al. Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering (asymptomatic) multiple myeloma. Blood. 2008;111:785–789. doi: 10.1182/blood-2007-08-108357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pratt G. The evolving use of serum free light chain assays in haematology. Brit J Haematol. 2008;141:413–422. doi: 10.1111/j.1365-2141.2008.07079.x. [DOI] [PubMed] [Google Scholar]
- 28.McLaughlin CL, Solomon A. A hidden antigenic site localized to the constant region of light chains of immunoglobulins. Science. 1973;179:580–582. doi: 10.1126/science.179.4073.580. [DOI] [PubMed] [Google Scholar]
- 29.Solomon A. Bence Jones proteins and light chains of immunoglobulins. XIV. Conformational dependency and molecular localization of the kappa (κ) and lambda (λ) antigenic determinants. Scand J Immunol. 1976;5:685–695. doi: 10.1111/j.1365-3083.1976.tb03018.x. [DOI] [PubMed] [Google Scholar]
- 30.Solomon A, McLaughlin CL, Steinberg AG. Bence Jones proteins and light chains of immunoglobulins. III. Inv antigenicity: a genetic expression with serologic dependency on the intact kappa light chain molecule. Immunochemistry. 1970;7:709–713. doi: 10.1016/0019-2791(70)90177-1. [DOI] [PubMed] [Google Scholar]
- 31.Coriu D, Weaver K, Schell M, Eulitz M, Murphy CL, Weiss DT, Solomon A. A molecular basis for nonsecretory myeloma. Blood. 2004;104:829–831. doi: 10.1182/blood-2004-02-0477. [DOI] [PubMed] [Google Scholar]
- 32.Bradwell AR, Carr-Smith HD, Mead GP, et al. Highly sensitive automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem. 2001;47:673–680. [PubMed] [Google Scholar]
- 33.Katzman JA, Clark RJ, Abraham RS, et al. Serum reference intervals and diagnostic ranges for free κ and free λ immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem. 2002;48:1437–1444. [PubMed] [Google Scholar]
- 34.Tate JR, Gill D, Cobcroft R, et al. Practical considerations for the measurement of free light chains in serum. Clin Chem. 2003;49:1957–1958. [Google Scholar]
- 35.Daval S, Tridon A, Mazeron N, et al. Risk of antigen excess in serum free light chain measurements. Clin Chem. 2007;53:1985–1986. doi: 10.1373/clinchem.2007.093377. [DOI] [PubMed] [Google Scholar]
- 36.Tate JR, Mollee M, Dimeski G, et al. Analytical performance of serum free light-chain assay during monitoring of patients with monoclonal light-chain diseases. Clin Chim Acta. 2007;376:30–36. doi: 10.1016/j.cca.2006.07.011. [DOI] [PubMed] [Google Scholar]