Abstract
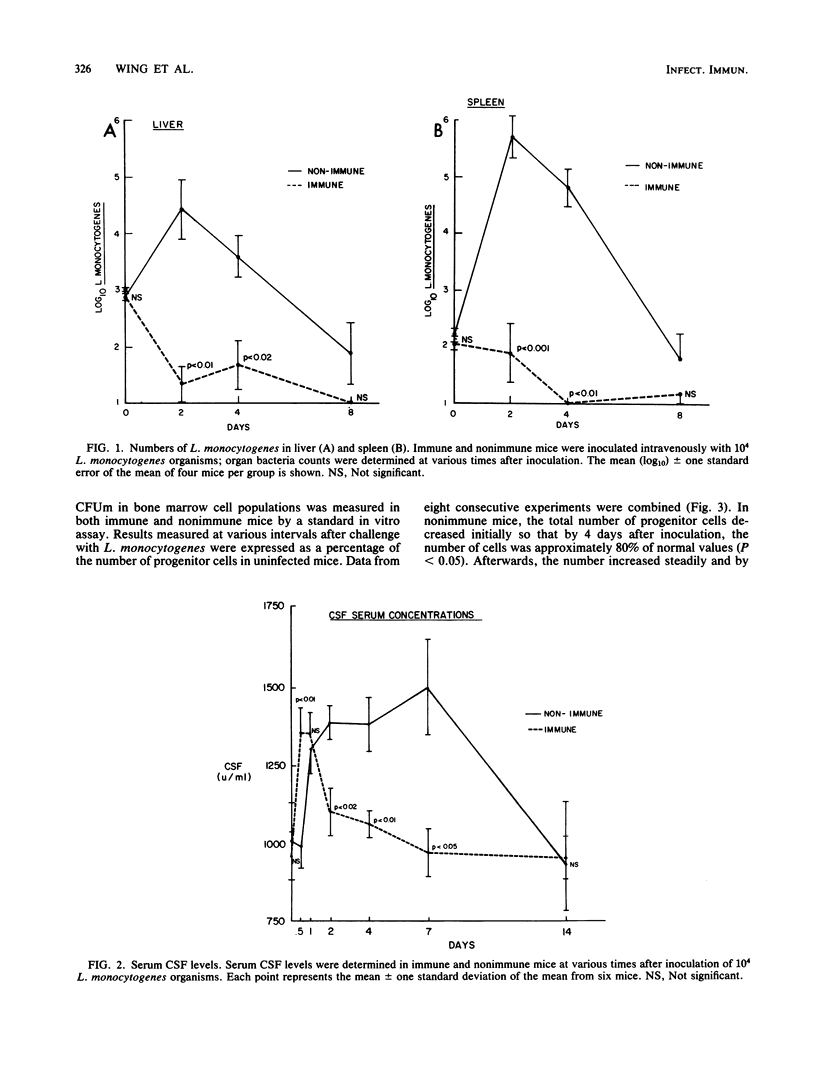
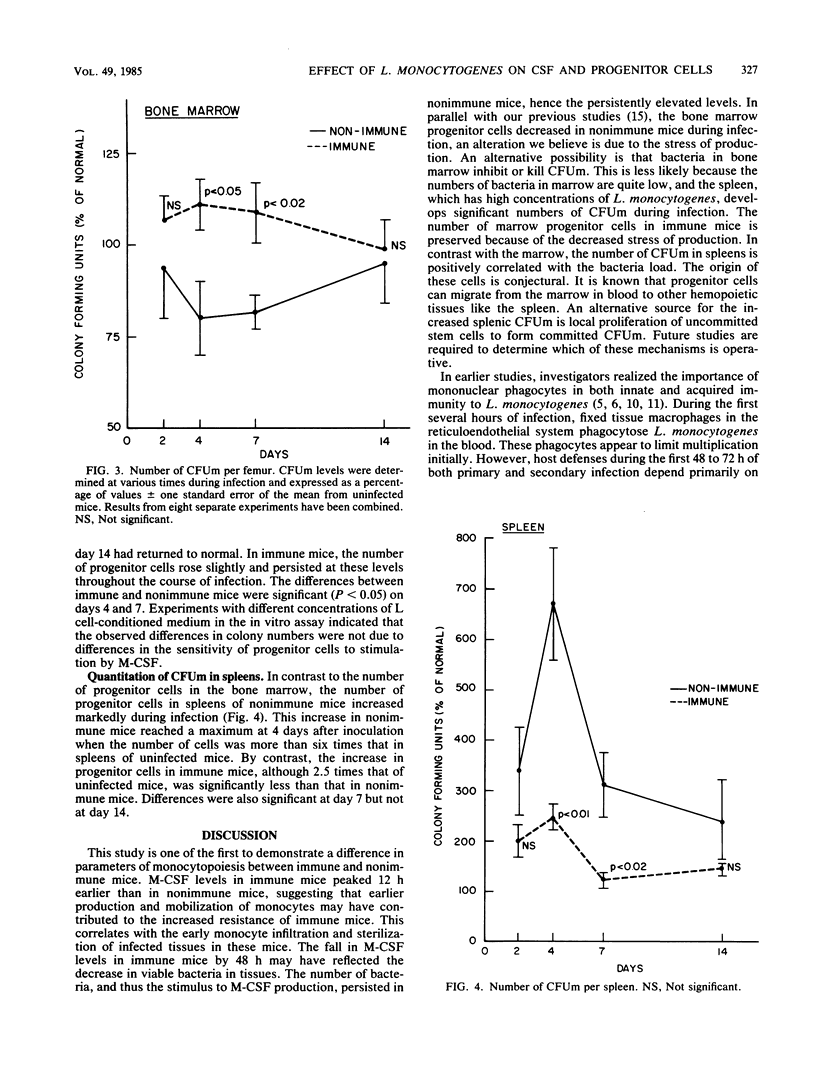
Studies were performed to determine changes in serum macrophage colony-stimulating factor (M-CSF) levels and the number of macrophage progenitor cells in bone marrow and spleens of nonimmune and immune mice infected with Listeria monocytogenes. Immunity in mice was established by infecting mice 6 weeks before use with a sublethal dose of L. monocytogenes. When challenged with 10(4) L. monocytogenes organisms, immune mice had an early (12 h) peak in M-CSF serum concentrations. Levels remained elevated for 24 h but fell towards normal by 48 h. By contrast, M-CSF levels in nonimmune mice did not rise until 24 h after challenge, remained elevated for 7 days, and returned to normal by 14 days. The number of macrophage progenitor cells in the bone marrow of immune mice rose slightly during infection, whereas the number in nonimmune mice fell significantly by days 4 and 7. Progenitor cells in spleens of immune mice more than doubled during infection; in nonimmune mice, a sixfold increase was noted. These results indicate that important parameters of monocyte production differ in immune and nonimmune mice during listeria infection and suggest a possible mechanism for differences in resistance to infection.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett M., Baker E. E. Marrow-dependent cell function in early stages of infection with Listeria monocytogenes. Cell Immunol. 1977 Sep;33(1):203–210. doi: 10.1016/0008-8749(77)90147-2. [DOI] [PubMed] [Google Scholar]
- Boegel F., Waheed A., Shadduck R. K. Evaluation of radioimmunoassay and in vitro colony assay techniques for determination of colony-stimulating factor and inhibitory activity in murine serum and tissue. Blood. 1981 Dec;58(6):1141–1147. [PubMed] [Google Scholar]
- Hahn H. Requirement for a bone marrow-derived component in the expression of cell-mediated antibacterial immunity. Infect Immun. 1975 May;11(5):949–954. doi: 10.1128/iai.11.5.949-954.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor D. D., Koster F. T. The mediator of cellular immunity. IV. Cooperation between lymphocytes and mononuclear phagocytes. Cell Immunol. 1971 Aug;2(4):317–325. doi: 10.1016/0008-8749(71)90066-9. [DOI] [PubMed] [Google Scholar]
- Metcalf D. Regulation of macrophage production. Adv Exp Med Biol. 1982;155:33–48. doi: 10.1007/978-1-4684-4394-3_3. [DOI] [PubMed] [Google Scholar]
- Newborg M. F., North R. J. On the mechanism of T cell-independent anti-Listeria resistance in nude mice. J Immunol. 1980 Feb;124(2):571–576. [PubMed] [Google Scholar]
- North R. J. Suppression of cell-mediated immunity to infection by an antimitotic drug. Further evidence that migrant macrophages express immunity. J Exp Med. 1970 Sep 1;132(3):535–545. doi: 10.1084/jem.132.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näher H., Sperling U., Hahn H. H-2K-restricted granuloma formation by Ly-2+ T cells in antibacterial protection to facultative intracellular bacteria. J Immunol. 1985 Jan;134(1):569–572. [PubMed] [Google Scholar]
- Shum D. T., Galsworthy S. B. Stimulation of monocyte production by an endogenous mediator induced by a component from Listeria monocytogenes. Immunology. 1982 Jun;46(2):343–351. [PMC free article] [PubMed] [Google Scholar]
- Sluiter W., van Waarde D., van Furth R. Regulation of monocyte precursor cell proliferation by two endogenous factors. Immunobiology. 1982 Apr;161(3-4):219–226. doi: 10.1016/S0171-2985(82)80077-6. [DOI] [PubMed] [Google Scholar]
- Wing E. J., Kresefsky-Friedman D. Y. Decreased resistance to Listeria monocytogenes in mice injected with killed corynebacterium parvum: association with suppression of cell-mediated immunity. J Infect Dis. 1980 Feb;141(2):203–211. doi: 10.1093/infdis/141.2.203. [DOI] [PubMed] [Google Scholar]
- Wing E. J., Waheed A., Shadduck R. K. Changes in serum colony-stimulating factor and monocytic progenitor cells during Listeria monocytogenes infection in mice. Infect Immun. 1984 Jul;45(1):180–184. doi: 10.1128/iai.45.1.180-184.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]



