Abstract
The frequent presence of deoxynivalenol (DON) in cereal-based foods and the high intake of these foods by children raises particular concerns about the relative susceptibility of this subpopulation to adverse effects evoked by this mycotoxin. We tested the hypothesis that both toxicokinetics and proinflammatory cytokine gene expression following a oral DON exposure at 5 mg/kg bw differ between weanling (3–4 wk) and young adult (8–10 wk) female mice. DON was rapidly taken up with maximum plasma concentrations reaching 1.0 µg/ml in adult mice at 15 min, whereas DON levels were approximately twice as much in weanling mice at these times. DON was rapidly cleared in both weanling and adult mice with concentrations being reduced by 78 and 81% of the peak levels, respectively, after 2 h. DON accumulation and clearance in spleen, liver, lung and kidney followed similar kinetics to that of plasma with tissue burdens also reaching twice that of adult mice. When TNF-α, IL-1β and IL-6 mRNAs in spleens (a primary source of systemic proinflammatory cytokines) were used as biomarkers of the DON’s effects, expression of these mRNAs was two to three times greater in weanling than adult mouse. However, differences in proinflammatory cytokine expression were less robust or not apparent in the liver or lung. Taken together, these data suggest that young mice are modestly more susceptible than adult mice to the adverse effects of DON and that this might result from a greater toxin tissue burden.
Keywords: Deoxynivalenol, Trichothecene, Mycotoxin, Age, Toxicokinetics, Cytokine
Introduction
The trichothecene deoxynivalenol (DON), colloquially known as vomitoxin, occurs frequently in wheat and barley worldwide as a result of outbreaks of Fusarium head blight (McMullen et al. 1997; Yuen and Schoneweis 2007). DON is of public health concern because of its: (1) often unavoidable capacity to contaminate agricultural commodities (Abouzied et al. 1991; Schothorst and van Egmond 2004), (2) recalcitrance to degradation during milling or processing (Hazel and Patel 2004) and (3) potential to cause human and animal toxicity (Pestka and Smolinski 2005).
Numerous toxicologic studies of DON have been conducted in several animal species to elucidate this toxin’s tissue targets, mechanisms of action and predict the threshold doses for potential adverse effects in humans. These data have been used to establish tolerable daily intakes (TDIs) and regulatory limits in specific foods. (Canady et al. 2001; Pieters et al. 2002). Two of the most sensitive toxicologic endpoints for DON are impaired growth and altered immune function (Pestka and Smolinski 2005).
Our laboratory has demonstrated that DON activates mitogen-activated protein kinases (MAPKs), which in turn, drive expression of proinflammatory cytokines and chemokines. DON-induced MAPK activation and cytokine upregulation been observed in the mouse spleen and Peyer’s patches (Azcona-Olivera et al. 1995; Zhou et al. 1997) as well as in vitro and ex vivo cultures containing murine and human mononuclear phagocytes. (Islam et al. 2006; Zhou et al. 2003b; Zhou et al. 2005). Among the cytokines upregulated by DON, TNF-α, IL-1 β and IL-6 are well-known to aberrantly affect food intake, growth and immune function (Borish and Steinke 2003; Johnson 1998). Proinflammatory cytokines might thus serve as sensitive biomarkers of effect that are predictive of DON’s adverse effects in experimental animals.
Several surveys suggest that DON is a frequent contaminant of infant food. Lombaert et al. (Lombaert et al. 2003) analyzed 363 cereal-based infant foods for mycotoxins and detected DON in 63% of the samples. A European survey of foods from 12 countries found that of a total of 11,022 samples analyzed for DON, 57% were positive for this trichothecene (Schothorst and van Egmond 2004). When these latter data were used to estimate mean intakes of this toxin by children, the values closely approached the TDI which represents the no adverse effect level divided by a 100-fold safety factor (Canady et al., 2001). Furthermore, children with high estimated intakes (≥ 95 percentile) were exposed to DON at levels exceeding the TDI. In an in-house survey of diets consumed by young children (Schothorst et al. 2005), it was found that, for 9 out of 74 respondents, consumption of DON by children exceeded the TDI. Children could consume more DON than adults and thus be at higher risk.
The issue of childhood exposure to DON is further complicated by the possibility that children might be inherently more sensitive to DON than adults. Several maturational factors can alter an individual’s response to a xenobiotic (Dourson et al. 2002). Specifically, age-related differences in gastrointestinal uptake, disposition, metabolism and renal excretion of xenobiotics have been described (Strolin et al. 2005). In addition, children will have a greater caloric need than the adults on a bodyweight basis and therefore might consume more DON on a body weight basis. The net effect of such differences might be an elevated tissue burden of DON and increase in toxic sequelae. To our knowledge, the question of whether such age-related differences exist in DON sensitivity has not been systematically addressed.
The purpose of this research was to test the hypothesis that both toxicokinetics and proinflammatory cytokine gene expression following acute DON exposure differ in weanling and adult mice. The results indicate that DON plasma and tissue concentrations were greater in weanling mice than adult mice. DON induction of IL-6 and TNF-α was higher and more prolonged in spleens (a primary source of systemic cytokines) of weanling mice than those of adult mice, whereas such differences were not obvious in the liver or lung.
Materials and Methods
Animals
All animal studies were conducted according to National Institutes of Health guidelines as overseen by the All University Committee on Animal Use and Care at Michigan State University. Weanling (3–4 wk) and young adult (8–10 wks) pathogen-free female B6C3F1 mice (Charles River, Portage, MI) were randomly assigned to experimental groups and housed in polycarbonate boxes containing Cell-Sorb Plus bedding (A & W Products, Cincinnati, OH), and covered with filter bonnets. Mice were housed 3 per cage under a 12 h light/dark cycle, and provided standard rodent chow and water ad libitum. Room lights were set on a 12 h light/dark cycle, and temperature and relative humidity were maintained between 21–24°C and 40–55% humidity, respectively.
Experimental design
For each experiment, groups of mice (n≥ 5) were gavaged with 5 mg/kg bw DON (Sigma Chemical Co., St. Louis, MO), dissolved in 100 µl of Dulbecco’s phosphate buffered saline (PBS) (Sigma-Aldrich, St Louis, MO), using a 22 G intubation needle (Popper and Sons, New Hyde Park, NY). This dose was used because, it is suboptimal for inducing cytokine expression in mice (Azcona-Olivera et al. 1995; Zhou et al. 1997) and thus facilitates detection of up- or down-regulated responses. After selected time intervals, mice were deeply anesthetized by i.p. injection with 0.1 ml of 12% (w/v) sodium pentobarbital in saline at designated post-exposure time intervals (0, 15, 30, 60 and 120 min). The abdominal cavity was opened and blood was collected via the caudal vena cava, and stored in EDTA-containing tubes. Following blood collection, other tissues were collected. For each mouse, left lung lobe, cranial half of spleen, lateral lobe of liver and right kidney were collected for DON analysis, while cardiac lobe of lung, caudal half of spleen and medial lobe of liver were collected for measurement of cytokine mRNA expression.
DON quantitation
Prior to DON measurement, plasma was prepared from blood by centrifugation and stored at −20°C. Organs (40–200 mg) were homogenized in PBS (1:10 [w/v]), and the homogenate centrifuged at 15,000 × g for 10 min. The supernatant fraction was first heated at 100EC for 5 min to inactivate endogenous enzymes and precipitate proteins; and then centrifuged at 15,000 × g for 10 min. The resultant supernatant was used with appropriate dilution for DON analysis.
DON was quantitated using a Veratox High Sensitivity (HS) ELISA (Neogen, Lansing, MI) according to manufacturer’s instruction with some modifications. Briefly, DON horseradish peroxidase conjugate was diluted (1:7 [v/v]) in 1% (w/v) bovine serum albumin (Sigma) in PBS. One hundred microliter (100 ul) aliquots of DON standards (1–200 ng/ml) or appropriately diluted samples were mixed with 100 µl of diluted enzyme conjugates and then incubated in antibody-coated microtiter wells for 45 min. After incubation, wells were aspirated and washed with distilled water. DON HS substrate (100 ul) was added and further incubated for 20 min. The reaction was terminated by adding 100 µl of stop reagent and plates read at 690 nm on an ELISA plate reader (Molecular Devices, Menlo Park, CA). DON concentrations in samples were determined from the standard curve using Softmax software (Molecular Devices). Using this method, we have previously determined that, DON recoveries of 90% or higher were obtained in heated liver extracts spiked directly with the toxin at 10–250 ng/g tissue and in liver homogenates that were spiked with the toxin at 10–250 ng/g, heated and supernatants analyzed (Pestka et al., 2008).
Toxicokinetic analysis
A two-compartment open model (Shargel et al. 2004) was employed to calculate pharmacokinetic parameters. DON concentrations in plasma and tissue were fitted to biexponential expression to calculate clearance rates (Li et al. 1997). Briefly, using the Trapezoidal rule, plasma area under the curve (AUC0-∞)=A/α+B/β, where A and B are y-intercepts of distribution and elimination curves respectively; while α and β are slopes for distribution and elimination curves respectively.
Real-time PCR for proinflammatory cytokine mRNAs
Excised tissues for PCR analyses were stored immediately after harvesting in RNAlater™ (Ambion Inc., Austin, TX). RNA was isolated using Tri Reagent (Molecular Research Center, Inc, Cincinnati, OH). Real-time PCR for IL-1β, TNF-α, IL-6 were performed on an ABI PRISM® 7900HT Sequence Detection System, using Taqman One-Step Real-time PCR Master Mix and Assays-on-Demand™ primer/probe gene expression products according to the manufacturer’s protocols (Applied Biosystems, Foster City, NY). Relative quantification of proinflammatory cytokine gene expression was carried out using β2-microglobulin RNA control and an arithmetic formula method (Audige et al. 2003; Islam et al. 2006).
Statistics
All data were analyzed with SigmaStat v 3.1 (Jandel Scientific, San Rafael,CA) with the criterion for significance set at p<0.05. Student’s t-test was used to compare differences between two groups of data. Two-way ANOVA was used for comparison of multiple groups. If the normality test passed, the Holm-Sidak method was used for post-hoc analysis; otherwise Dunnett’s test was used.
Results
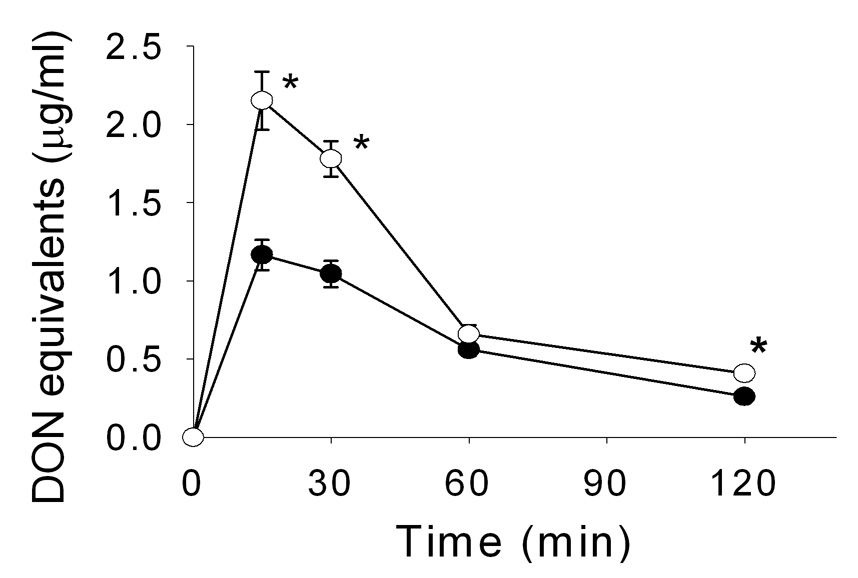
DON plasma and tissue concentrations in weanling and adult mice were compared at 15, 30, 60 and 120 min after oral exposure to 5 mg/kg of the toxin. DON was rapidly taken up in the adult mouse and plasma concentrations found to be maximal (approximately 1.0 µg/ml) at 15 and 30 min (Fig. 1). In contrast, DON concentrations in weanling mice were approximately twice that found for adult mice at 15 and 30 min. DON was rapidly cleared in weanling and adult mice with concentrations being reduced by 78 and 81% of the peak levels, respectively, after 2 h. The plasma AUC for weanling mice was 4.38 µg × h/ml, while in adult mice it was 3.15 µg × h/ml.
Figure 1. Time-dependent changes in DON concentration in plasma of adult (solid circles) and weanling (open circles) mice following a single oral exposure to 5 mg/kg of DON.
Data are mean ± SEM (n≥8). Asterisk indicates significant difference (p<0.05) between the two groups at the corresponding timepoint.
The kinetics of DON accumulation in the four organs were similar to that of plasma. DON concentrations at 15, 30, 60 and 120 min were significantly higher in spleens (Fig. 2A) and lungs (Fig. 2B) of weanling mice than adult mice. DON also distributed into the liver and reached peak concentrations in the adult of 1 µg/g at 30 and 60 min (Fig. 2C). DON concentrations were significantly higher in lungs of weanlings at 15 min but not 30, 60 and 90 min. The kidney attained the highest DON concentrations of all organs tested, reaching 1.8 µg/g at 15 min in adult mice while DON levels in weanling kidneys were approximately twice that much (Fig. 2D). Kidney DON concentrations were significantly higher in weanling mice at 15, 30 and 120 min. Collectively, these data show that DON distributed into plasma and tissues to a greater extent in weanling mice than in adult mice.
Figure 2. Time-dependent changes in DON concentration in spleen, lung, liver and kidney of adult (solid circles) and weanling (open circles) mice following a single oral exposure to 5 mg/kg of DON.
Data are mean ± SEM (n≥8). Asterisk indicates significant difference (p<0.05) between the two groups at the corresponding timepoint.
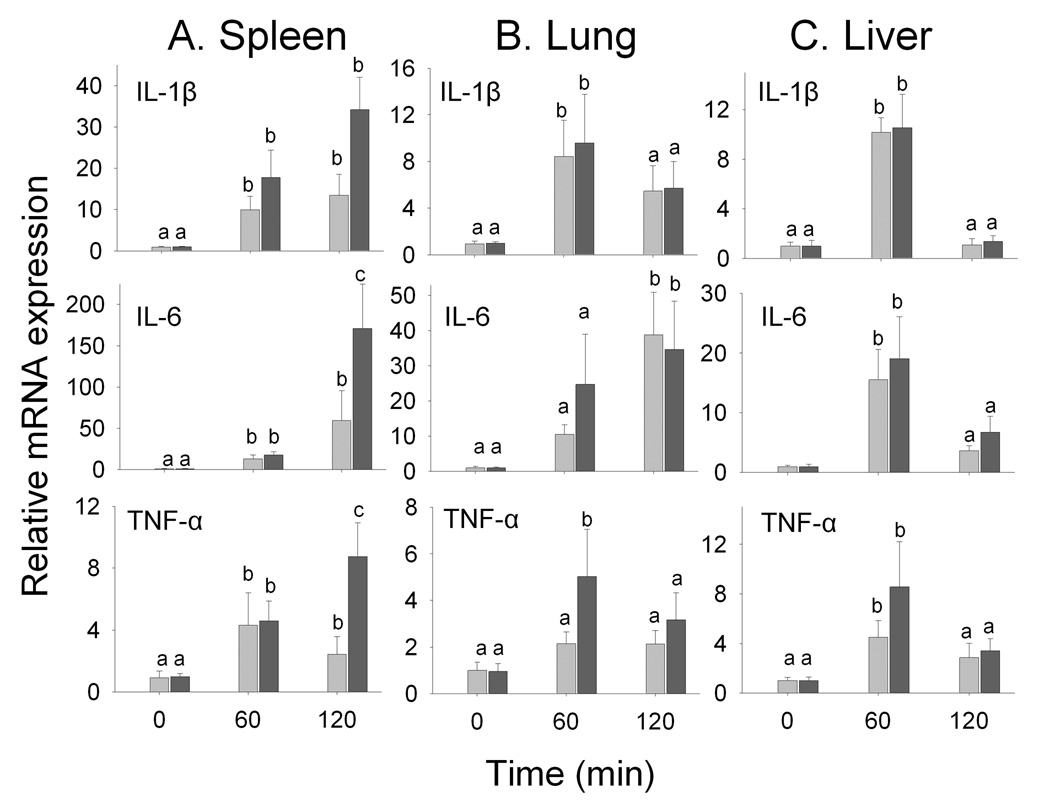
Proinflammatory cytokine mRNAs were employed as biomarkers of effect to address whether the same DON dose differentially affects weanling and adult mice. DON induced robust expression of IL-1β, IL-6 and TNF-α mRNAs in spleens of adult mice at 60 and 120 min (Fig. 3A). While comparable effects were observed for weanling mice at 60 min, both IL-6 and TNF-α mRNA expression were significantly higher in weanling mice at 120 min with a similar trend being observed for IL-1β.
Figure 3. Time-dependent changes in proinflammatory cytokine mRNA expression in spleen, lung, liver and kidney of adult (light bar) and weanling (dark bar) mice following a single oral exposure to 5 mg/kg of DON.
Data are mean ± SEM (n≥8). Data are mean ± SEM (n≥8). Bars labeled (a) are significantly higher than control (0 min) whereas bars labeled (b) are significantly higher than orally exposed mice at the same time point (p<0.05).
DON also induced proinflammatory cytokines in lung and liver but to a lesser extent than observed in spleen. Specifically, DON-induced IL-1β mRNA expression was similar in the lungs of weanling and adult mice at 60 min with a comparable trend being evident at 120 min (Fig. 3B). IL-6 mRNA was elevated at 120 min in weanling and adult lungs with no differences detectable between the groups. Finally, TNF-α mRNA in lung of weanling mice was elevated at 60 min in response to DON whereas all other groups were unaffected. Expression of IL-1β, IL-6 and TNF-α mRNAs was significantly increased in livers of both weanling and adult mice at 60 min but not 120 min (Fig. 3C). However, no differences in liver responses were observed at between the weanling and adult.
Discussion
The potential for chemicals to differentially affect human subpopulations is an important consideration when determining TDIs and establishing regulatory standards for natural toxins (Falk-Filipsson et al. 2007). Children are one population of special interest because they are recognized to handle uptake and clearance of some xenobiotics differently than adults (Faustman et al. 2000). The higher intake of DON-contaminated cereal-based foods by children as compared to adults (Pieters et al. 2002; Schothorst et al. 2005) further elevates concerns about risks of adverse effects in this human subpopulation. In this study, weanling mice were used to mimic 1–2 yr in humans, representing the beginning of independence from dam as well as onset of rapid growth. The results presented herein indicate that, when given equivalent doses, DON uptake in plasma and organs of weanling mice was approximately twice that observed in the young adult mice. Furthermore, induction of proinflammatory cytokines, indicators of DON’s toxic effects in weanling mice were significantly higher and more prolonged in spleen but not in liver or lung.
Our laboratory previously utilized 3[H]-DON to assess the distribution and clearance of DON from 30 min to 24 h in adult mice orally exposed to 5 mg/kg bw of the toxin (Azcona-Olivera et al. 1995). Plasma and organ distribution of DON were found to be directly proportional to the two doses employed (5 and 25 mg/kg bw). Two compartment kinetics were observed in that study, with an initial rapid clearance rate and slower terminal elimination in plasma following exposure to DON at 5 mg/kg bw (t1/2α = 21.6 min and t1/2β = 7.6 h, respectively. The present study similarly indicated two compartment kinetics with the initial half-lives of 25.4 and 41 min being observed here for weanling and adult mice, respectively. DON concentrations observed in plasma at 30, 60 and 120 min following acute oral DON exposure at 5 mg/kg bw (1300, 740 and 370 ng/ml, respectively) in the isotope study were remarkably similar to those found here in adult mice (1040, 560 and 260 ng/ml, respectively). Also, mean DON concentrations in spleen at 30, 60 and 120 min following a 5 mg/kg dose in the radiolabel study (680, 210 and 120 ng/g, respectively) were comparable to those observed in this study for the adult mouse (710, 340 and 180 ng/ml, respectively). Furthermore, DON concentrations in the liver and kidney in the isotope investigation mirrored those found here. Taken together, these results indicate that ELISA data in the adult mouse were comparable to 3[H]-DON results, thus indicating the validity of using this immunoassay for comparing DON toxicokinetics in experimental animals.
DON evokes rapid and dose-dependent TNF-α, IL-6 and IL-1β mRNA upregulation in spleen (Azcona-Olivera et al. 1995; Zhou et al. 1997). These proinflammatory cytokines potentially contribute to anorectic, growth and immunologic effects (Borish and Steinke 2003; Johnson 1998), that are consistent with DON’s effects (Pestka and Smolinski, 2005). Therefore their expression can be employed as a dosimeter for DON toxicity in mice. Mitogen-activated protein kinase (MAPK)-driven activation of transcription factors and enhanced mRNA stability are known to mediate DON-induced cytokine expression (Pestka et al. 2004). Oral DON exposure sequentially induces: (1) phosphorylation of JNK 1/2, ERK 1/2 and p38 (15–30 min), (2) transcription factor activation (1–2 h), and (3) cytokine mRNA expression (1–4 h) (Zhou et al. 2003a) in spleen. The finding that peak DON concentrations in plasma and spleen occurred at 15 min is highly consistent with the kinetics of MAPK activation and subsequent robust proinflammatory gene expression seen here and previously. The additional observation that DON concentrations in plasma and organs in younger mice were nearly twice that of older mice correlates closely with the observation that splenic proflammatory gene upregulation 2 h after DON exposure in weanlings was two to three times that of the adults. Given the rapid uptake of DON in tissue and the further observation that the cytokine response was prolonged in young mice, it will be important to understand in the future how kinetics of DON-induced proinflammatory cytokine responses might be differentially affected by age.
As observed previously (Azcona-Olivera et al. 1995), DON induced modest expression of TNF-α, IL-6 and IL-1β in liver relative to spleen. However, major differences in responses of these tissues did not exist between weanling and adult mice. The observation that the spleen evoked a higher response to DON compared than liver or lung might reflect the presence of different sensitive cell populations in these tissues. While the liver is often thought to be a major source of proinflammatory cytokines, our laboratory has shown that the induction of TNF-α, IL-6 and IL-1β mRNA expression in the mouse in response to the prototypical inflammagen, lipopolysaccharide, is 3- to 20- times higher in spleen than liver (Pestka and Smolinski 2005). We are not aware of similar comparisons between spleen and lung. Nevertheless, it is still difficult as yet to reconcile the lack of correlation between heightened DON levels in liver and lung in weanling mice and proinflammatory cytokine responses. An additional limitation of this research is that cytokine expression was not measured in the kidney. This would have been of particular interest because of the high concentrations of toxin being found in that organ. Further research is therefore warranted on the comparative contributions of cytokine responses to DON in different organs and the toxin’s anorectic, growth and immunologic effects.
The specific mechanisms that underlay the modestly elevated DON tissue burdens in weanling mice observed here are not known. One possibility is that there is increased uptake from the gastrointestinal tract in the young animal because of increased transport or decreased gut metabolism of the toxin (Strolin et al. 2005). Regarding the latter, the the deepoxy metabolite of DON (DOM-1) results from metabolism by intestinal microbes (Swanson et al. 1987). DOM-1 is detectable in cow and sheep urine (Cote et al. 1987; Prelusky et al. 1987). DOM-1 is much less toxic than DON in vitro (Sundstol et al. 2004), indicating that deepoxidation is a true detoxification reaction. Such deepoxidation activity is observable in feces of rodents and swine (Danicke et al. 2004; Worrell et al. 1989) but not that of humans (Sundstol and Pettersson 2003). Clarification is thus needed on whether differences exist between weanling and adult mice in deepoxidation and, ultimately, the uptake of DON.
Another plausible explanation for the observed age effects is that that liver metabolism in weanling mice differs from that of the adult mice (Benedetti et al. 2007). By the first year of age, phase I and phase II metabolic activity is developing in humans and thus liver metabolites in children can differ from those of adults (Makri et al. 2004). A major hepatic detoxification metabolite of DON results from its conjugation to a glucuronide via mammalian UDP-glucuronosyl-transferase. In rats, approximately one third of administered DON is excreted in the urine, with DON-glucuronide being the major urinary metabolite (Meky et al. 2003). Glucuronidation of DON renders it non-toxic (Wu et al. 2007). DON glucuronide is also detectable in human urine (Meky et al. 2003); this metabolite has recently been employed as biomarker of human exposure (Turner et al. 2007). The possibility exists that weanlings were less capable than adults in forming the glucuronide metabolite and therefore in rendering DON less toxic and clearing it from the body. However, DON levels in weanlings dropped to near adult levels within the 120 min suggesting that weanlings were similar to adults in their ability to clear DON from tissues. Thus age-related differences might be more pronounced for DON uptake than for clearance. There is need for additional investigation of whether weanling and adult mice differ in their DON glucuronidation activities and how this might impact the tissue burden.
Taken together, the results presented herein indicate that plasma and tissue DON concentrations in weanling mice were approximately twice that of adult mice given identical doses of the toxin. DON induction of TNF-α, IL-1β and IL-6 in young animals were two to three times greater in spleens, a primary source of systemic proinflammatory cytokines, whereas differences in proinflammatory cytokine expression were less robust or not apparent in the liver or lung between the two age groups. It is not readily apparent how these effects might impact TDI estimates. Since the effects were indeed quite modest, it might be speculated that they would be accounted for when the conventional 10-fold safety factor for human variability is employed in TDI estimates. A limitation of this study is that the 5 mg/kg bw dose employed here represents the amount of DON a mouse would eat over a 1 d period in food contaminated with 35 ppm of the toxin. This concentration in food would reflect an extreme upper limit to levels found in food. To extrapolate these findings to humans, future studies need to compare the dose response effects of acute and short-term DON exposure on tissue burden and proinflammatory cytokine expression in weanling and adult mice. In addition, further comparative investigations are needed on how toxicokinetics and proinflammatory cytokine biomarkers might be modulated when murine models for the neonatal and elderly are challenged with DON.
Acknowledgements
This work was supported in part by the USDA, under a cooperative project with U.S. Wheat and Barley Scab Initiative. Any findings, opinions, conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of USDA. This project was also funded in part by Public Health Service Grant ES 3358 (JJP) from the National Institute for Environmental Health Sciences. We would like to thank Dr. Zahidul Islam, Dr. Maoxiang Li, Eleni Beli and Mary Rosner for technical assistance.
Abbreviations
- AUCs
areas under the curve
- DON
Deoxynivalenol
- HS
high sensitivity
- MAPKs
mitogen-activated protein kinases
- PBS
phosphate buffered saline
- TDIs
tolerable daily intakes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abouzied MM, Azcona JI, Braselton WE, Pestka JJ. Immunochemical assessment of mycotoxins in 1989 grain foods: evidence for deoxynivalenol (vomitoxin) contamination. Appl. Environ. Microbiol. 1991;57:672–677. doi: 10.1128/aem.57.3.672-677.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audige A, Yu ZR, Frey BM, Uehlinger DE, Frey FJ, Vogt B. Epithelial sodium channel (ENaC) subunit mRNA and protein expression in rats with puromycin aminonucleoside-induced nephrotic syndrome. Clin. Sci. 2003;104:389–395. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Azcona-Olivera JI, Ouyang Y, Murtha J, Chu FS, Pestka JJ. Induction of cytokine mRNAs in mice after oral exposure to the trichothecene vomitoxin (deoxynivalenol): relationship to toxin distribution and protein synthesis inhibition. Toxicol. Appl. Pharmacol. 1995;133:109–120. doi: 10.1006/taap.1995.1132. [DOI] [PubMed] [Google Scholar]
- Benedetti MS, Whomsley R, Canning M. Drug metabolism in the paediatric population and in the elderly. Drug Discov. Today. 2007;12:599–610. doi: 10.1016/j.drudis.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Borish LC, Steinke JW. Cytokines and chemokines. J. Allergy Clin. Immunol. 2003;111:S460–S475. doi: 10.1067/mai.2003.108. [DOI] [PubMed] [Google Scholar]
- Canady RA, Coker RD, Egan SK, Krska R, Kuiper-Goodman T, Olsen M, Pestka J, Resnik S, Schlatter J. Deoxynivalenol. WHO Food Addit. Ser.; WHO/IPCS Safety evaluation of certain mycotoxins in food. 2001;47:419–555.
- Cote LM, Buck W, Jeffery E. Lack of hepatic microsomal metabolism of deoxynivalenol and its metabolite, DOM-1. Food Chem. Toxicol. 1987;25:291–295. doi: 10.1016/0278-6915(87)90125-6. [DOI] [PubMed] [Google Scholar]
- Danicke S, Valenta H, Doll S. On the toxicokinetics and the metabolism of deoxynivalenol (DON) in the pig. Arch. Anim. Nutr. 2004;58:169–180. doi: 10.1080/00039420410001667548. [DOI] [PubMed] [Google Scholar]
- Dourson M, Charnley G, Scheuplein R. Differential sensitivity of children and adults to chemical toxicity. II. Risk and regulation. Regul. Toxicol. Pharmacol. 2002;35:448–467. doi: 10.1006/rtph.2002.1559. [DOI] [PubMed] [Google Scholar]
- Falk-Filipsson A, Hanberg A, Victorin K, Warholm M, Wallen M. Assessment factors--applications in health risk assessment of chemicals. Environ. Res. 2007;104:108–127. doi: 10.1016/j.envres.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Faustman EM, Silbernagel SM, Fenske RA, Burbacher TM, Ponce RA. Mechanisms underlying Children's susceptibility to environmental toxicants. Environ. Health Perspect. 2000;108 Suppl 1:13–21. doi: 10.1289/ehp.00108s113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazel CM, Patel S. Influence of processing on trichothecene levels. Toxicol. Lett. 2004;153:51–59. doi: 10.1016/j.toxlet.2004.04.040. [DOI] [PubMed] [Google Scholar]
- Islam Z, Gray JS, Pestka JJ. p38 Mitogen-activated protein kinase mediates IL-8 induction by the ribotoxin deoxynivalenol in human monocytes. Toxicol. Appl. Pharmacol. 2006;213:235–244. doi: 10.1016/j.taap.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Johnson RW. Immune and endocrine regulation of food intake in sick animals. Domest. Anim. Endocrinol. 1998;15:309–319. doi: 10.1016/s0739-7240(98)00031-9. [DOI] [PubMed] [Google Scholar]
- Li S, Marquardt RR, Frohlich AA, Vitti TG, Crow G. Pharmacokinetics of ochratoxin A and its metabolites in rats. Toxicol. Appl. Pharmacol. 1997;145:82–90. doi: 10.1006/taap.1997.8155. [DOI] [PubMed] [Google Scholar]
- Lombaert GA, Pellaers P, Roscoe V, Mankotia M, Neil R, Scott PM. Mycotoxins in infant cereal foods from the Canadian retail market. Food Addit. Contam. 2003;20:494–504. doi: 10.1080/0265203031000094645. [DOI] [PubMed] [Google Scholar]
- Makri A, Goveia M, Balbus J, Parkin R. Children's susceptibility to chemicals: a review by developmental stage. J. Toxicol. Environ. Health B Crit Rev. 2004;7:417–435. doi: 10.1080/10937400490512465. [DOI] [PubMed] [Google Scholar]
- McMullen M, Jones R, Gallenberg D. Scab of wheat and barley: a re-emerging disease of devastating impact. Plant Dis. 1997;81:1340–1348. doi: 10.1094/PDIS.1997.81.12.1340. [DOI] [PubMed] [Google Scholar]
- Meky FA, Turner PC, Ashcroft AE, Miller JD, Qiao YL, Roth MJ, Wild CP. Development of a urinary biomarker of human exposure to deoxynivalenol. Food Chem. Toxicol. 2003;41:265–273. doi: 10.1016/s0278-6915(02)00228-4. [DOI] [PubMed] [Google Scholar]
- Pestka JJ, Smolinski AT. Deoxynivalenol: toxicology and potential effects on humans. J. Toxicol. Environ. Health B Crit Rev. 2005;8:39–69. doi: 10.1080/10937400590889458. [DOI] [PubMed] [Google Scholar]
- Pestka JJ, Zhou HR, Moon Y, Chung YJ. Cellular and molecular mechanisms for immune modulation by deoxynivalenol and other trichothecenes: unraveling a paradox. Toxicol. Lett. 2004;153:61–73. doi: 10.1016/j.toxlet.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Pestka JJ, Islam Z, Amuzie CJ. Immunochemical assessment of deoxynivalenol tissue distribution following oral exposure in the mouse. Toxicol. Lett. 2008 doi: 10.1016/j.toxlet.2008.02.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters MN, Freijer J, Baars BJ, Fiolet DC, van KJ, Slob W. Risk assessment of deoxynivalenol in food: concentration limits, exposure and effects. Adv. Exp. Med. Biol. 2002;504:235–248. doi: 10.1007/978-1-4615-0629-4_25. [DOI] [PubMed] [Google Scholar]
- Prelusky DB, Veira DM, Trenholm HL, Foster BC. Metabolic fate and elimination in milk, urine and bile of deoxynivalenol following administration to lactating sheep. J. Environ. Sci. Health B. 1987;22:125–148. doi: 10.1080/03601238709372550. [DOI] [PubMed] [Google Scholar]
- Schothorst RC, Jekel AA, van Egmond HP, De MA, Boon PE, Van Klaveren JD. Determination of trichothecenes in duplicate diets of young children by capillary gas chromatography with mass spectrometric detection. Food Addit. Contam. 2005;22:48–55. doi: 10.1080/02652030400019414. [DOI] [PubMed] [Google Scholar]
- Schothorst RC, van Egmond HP. Report from SCOOP task 3.2.10 "collection of occurrence data of Fusarium toxins in food and assessment of dietary intake by the population of EU member states". Subtask: trichothecenes. Toxicol. Lett. 2004;153:133–143. doi: 10.1016/j.toxlet.2004.04.045. [DOI] [PubMed] [Google Scholar]
- Shargel L, Wu-Pong S, Yu ABC. Applied Biopharmaceutics and Pharmacokinetics. New York, NY: McGraw-Hill Medical; 2004. [Google Scholar]
- Strolin BM, Whomsley R, Baltes EL. Differences in absorption, distribution, metabolism and excretion of xenobiotics between the paediatric and adult populations. Expert. Opin. Drug Metab. Toxicol. 2005;1:447–471. doi: 10.1517/17425255.1.3.447. [DOI] [PubMed] [Google Scholar]
- Sundstol EG, Pettersson H. Lack of de-epoxidation of type B trichothecenes in incubates with human faeces. Food Addit. Contam. 2003;20:579–582. doi: 10.1080/0265203031000102573. [DOI] [PubMed] [Google Scholar]
- Sundstol EG, Pettersson H, Lundh T. Comparative cytotoxicity of deoxynivalenol, nivalenol, their acetylated derivatives and de-epoxy metabolites. Food Chem. Toxicol. 2004;42:619–624. doi: 10.1016/j.fct.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Swanson SP, Nicoletti J, Rood HD, Jr, Buck WB, Cote LM, Yoshizawa T. Metabolism of three trichothecene mycotoxins, T-2 toxin, diacetoxyscirpenol and deoxynivalenol, by bovine rumen microorganisms. J. Chromatogr. 1987;414:335–342. doi: 10.1016/0378-4347(87)80058-0. [DOI] [PubMed] [Google Scholar]
- Turner PC, Burley VJ, Rothwell JA, White KL, Cade JE, Wild CP. Dietary wheat reduction decreases the level of urinary deoxynivalenol in UK adults. J. Expo. Sci. Environ. Epidemiol. 2007 doi: 10.1038/sj.jes.7500611. in press. [DOI] [PubMed] [Google Scholar]
- Worrell NR, Mallett AK, Cook WM, Baldwin NC, Shepherd MJ. The role of gut micro-organisms in the metabolism of deoxynivalenol administered to rats. Xenobiotica. 1989;19:25–32. doi: 10.3109/00498258909034673. [DOI] [PubMed] [Google Scholar]
- Wu X, Murphy P, Cunnick J, Hendrich S. Synthesis and characterization of deoxynivalenol glucuronide: Its comparative immunotoxicity with deoxynivalenol. Food Chem. Toxicol. 2007;45:1846–1855. doi: 10.1016/j.fct.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Yuen GY, Schoneweis SD. Strategies for managing Fusarium head blight and deoxynivalenol accumulation in wheat. Int. J. Food Microbiol. 2007;119:126–130. doi: 10.1016/j.ijfoodmicro.2007.07.033. [DOI] [PubMed] [Google Scholar]
- Zhou HR, Islam Z, Pestka JJ. Rapid, sequential activation of mitogen-activated protein kinases and transcription factors precedes proinflammatory cytokine mRNA expression in spleens of mice exposed to the trichothecene vomitoxin. Toxicol. Sci. 2003a;72:130–142. doi: 10.1093/toxsci/kfg006. [DOI] [PubMed] [Google Scholar]
- Zhou HR, Jia Q, Pestka JJ. Ribotoxic stress response to the trichothecene deoxynivalenol in the macrophage involves the SRC family kinase Hck. Toxicol. Sci. 2005;85:916–926. doi: 10.1093/toxsci/kfi146. [DOI] [PubMed] [Google Scholar]
- Zhou HR, Lau AS, Pestka JJ. Role of double-stranded RNA-activated protein kinase R (PKR) in deoxynivalenol-induced ribotoxic stress response. Toxicol. Sci. 2003b;74:335–344. doi: 10.1093/toxsci/kfg148. [DOI] [PubMed] [Google Scholar]
- Zhou HR, Yan D, Pestka JJ. Differential cytokine mRNA expression in mice after oral exposure to the trichothecene vomitoxin (deoxynivalenol): dose response and time course. Toxicol. Appl. Pharmacol. 1997;144:294–305. doi: 10.1006/taap.1997.8132. [DOI] [PubMed] [Google Scholar]