Abstract
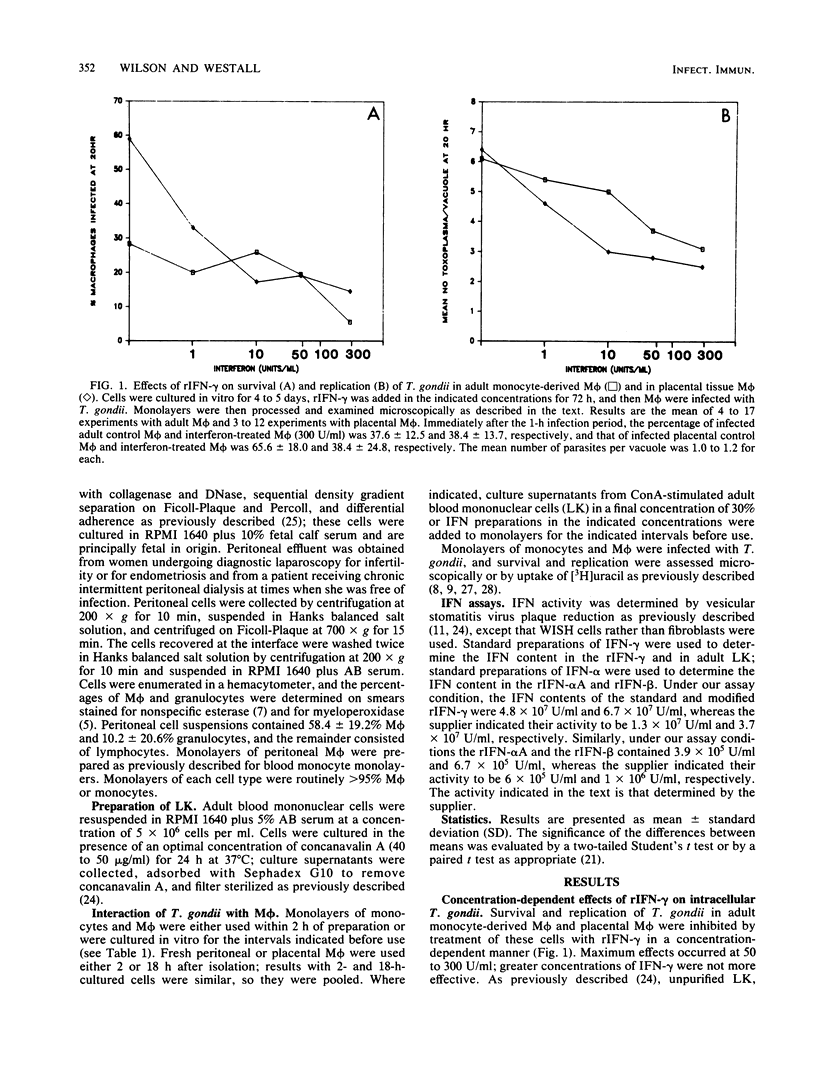
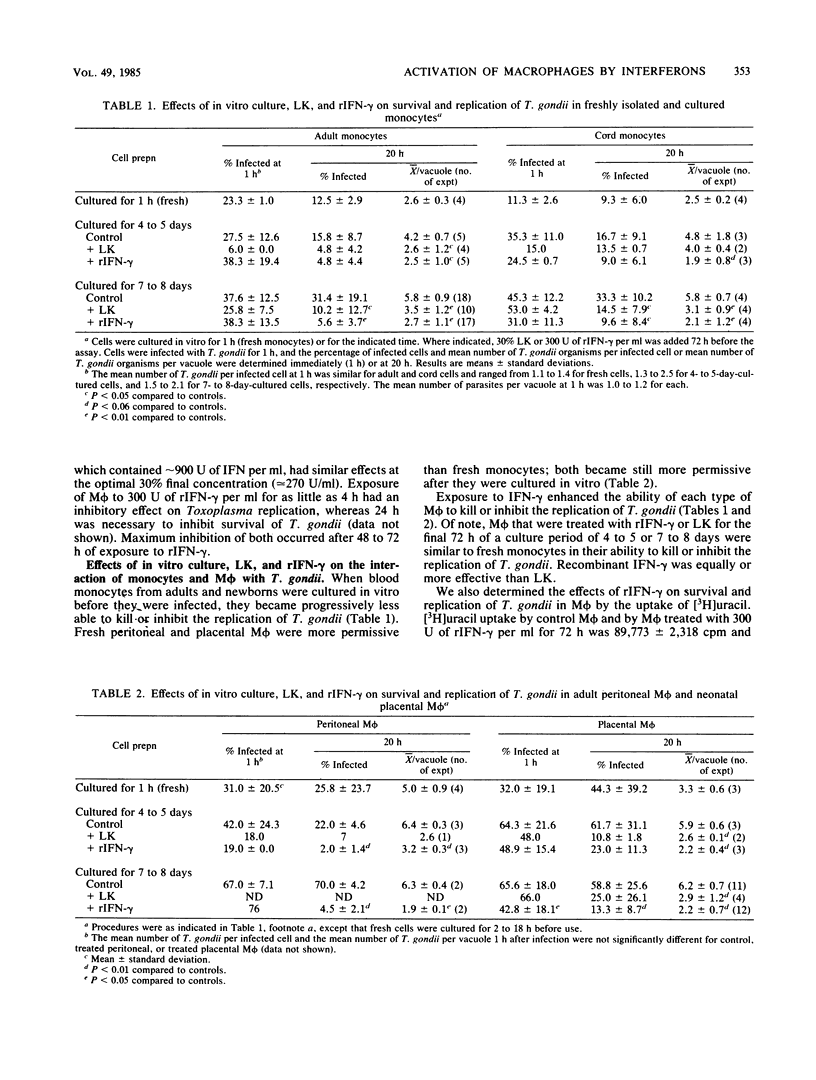
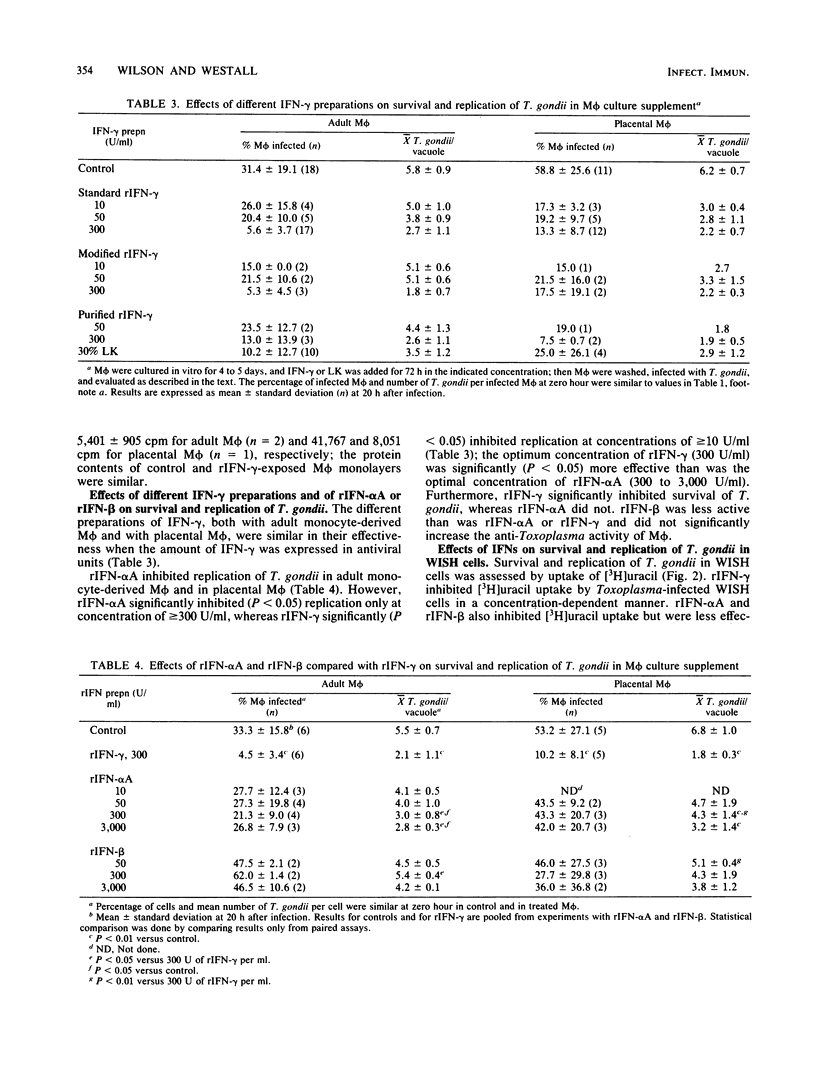
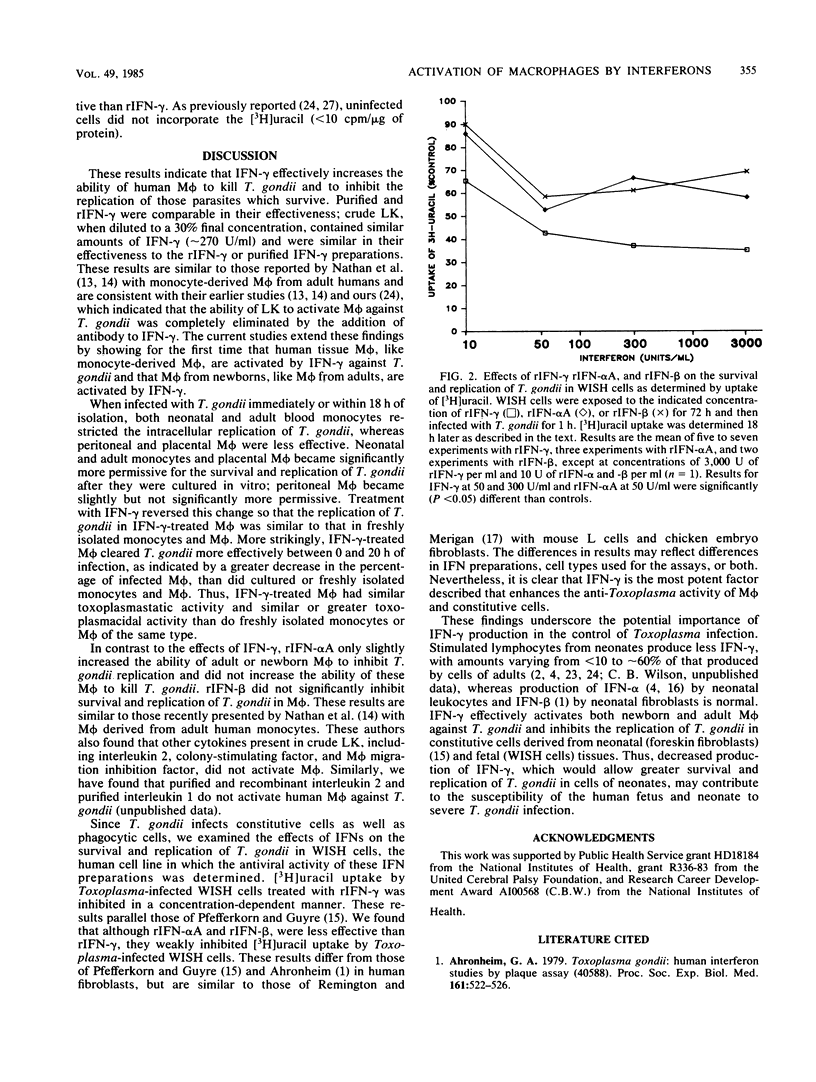
Increasing evidence suggests that gamma interferon (IFN-gamma) is the major or sole factor in human lymphokines which activates blood monocyte-derived macrophages (M phi) to inhibit or kill Toxoplasma gondii and certain other intracellular pathogens. In the current studies, we found that IFN-gamma effectively activated tissue M phi from adults (peritoneal M phi) and from newborns (placental M phi) as well as blood-derived M phi from adults and from newborns to kill or to inhibit the replication of T. gondii. Results with purified and recombinant IFN-gamma and with adult and newborn M phi were similar. IFN-gamma-treated M phi were equally or more active against T. gondii than were freshly isolated monocytes and M phi. Recombinant IFN-alpha A and IFN-beta were less effective than IFN-gamma. IFN-gamma also inhibited survival and replication of T. gondii in WISH cells more effectively than did IFN-alpha and IFN-beta. These findings are consistent with an important role for IFN-gamma in the control of Toxoplasma infection and indicate that the anti-Toxoplasma activity of resting and IFN-gamma-activated adult and neonatal M phi is similar. The increased susceptibility of neonates to T. gondii is not due to a defect in M phi effector function.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahronheim G. A. Toxoplasma gondii: human interferon studies by plaque assay. Proc Soc Exp Biol Med. 1979 Sep;161(4):522–526. doi: 10.3181/00379727-161-40588. [DOI] [PubMed] [Google Scholar]
- Bryson Y. J., Winter H. S., Gard S. E., Fischer T. J., Stiehm E. R. Deficiency of immune interferon production by leukocytes of normal newborns. Cell Immunol. 1980 Sep 15;55(1):191–200. doi: 10.1016/0008-8749(80)90150-1. [DOI] [PubMed] [Google Scholar]
- Gray P. W., Leung D. W., Pennica D., Yelverton E., Najarian R., Simonsen C. C., Derynck R., Sherwood P. J., Wallace D. M., Berger S. L. Expression of human immune interferon cDNA in E. coli and monkey cells. Nature. 1982 Feb 11;295(5849):503–508. doi: 10.1038/295503a0. [DOI] [PubMed] [Google Scholar]
- Handzel Z. T., Levin S., Dolphin Z., Schlesinger M., Hahn T., Altman Y., Schechter B., Shneyour A., Trainin N. Immune competence of newborn lymphocytes. Pediatrics. 1980 Mar;65(3):491–496. [PubMed] [Google Scholar]
- KAPLOW L. S. SIMPLIFIED MYELOPEROXIDASE STAIN USING BENZIDINE DIHYDROCHLORIDE. Blood. 1965 Aug;26:215–219. [PubMed] [Google Scholar]
- Kleinschmidt W. J., Schultz R. M. Similarities of murine gamma interferon and the lymphokine that renders macrophages cytotoxic. J Interferon Res. 1982;2(2):291–299. doi: 10.1089/jir.1982.2.291. [DOI] [PubMed] [Google Scholar]
- Locksley R. M., Wilson C. B., Klebanoff S. J. Role for endogenous and acquired peroxidase in the toxoplasmacidal activity of murine and human mononuclear phagocytes. J Clin Invest. 1982 May;69(5):1099–1111. doi: 10.1172/JCI110545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod R., Remington J. S. A method to evaluate the capacity of monocytes and macrophages to inhibit multiplication of an intracellular pathogen. J Immunol Methods. 1979 May 10;27(1):19–29. doi: 10.1016/0022-1759(79)90235-7. [DOI] [PubMed] [Google Scholar]
- Meltzer M. S., Benjamin W. R., Farrar J. J. Macrophage activation for tumor cytotoxicity: induction of macrophage tumoricidal activity by lymphokines from EL-4, a continuous T cell line. J Immunol. 1982 Dec;129(6):2802–2807. [PubMed] [Google Scholar]
- Meyers J. D., McGuffin R. W., Neiman P. E., Singer J. W., Thomas E. D. Toxicity and efficacy of human leukocyte interferon for treatment of cytomegalovirus pneumonia after marrow transplantation. J Infect Dis. 1980 May;141(5):555–562. doi: 10.1093/infdis/141.5.555. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Rothermel C. D. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes. Evidence that interferon-gamma is the activating lymphokine. J Clin Invest. 1983 Oct;72(4):1506–1510. doi: 10.1172/JCI111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Prendergast T. J., Wiebe M. E., Stanley E. R., Platzer E., Remold H. G., Welte K., Rubin B. Y., Murray H. W. Activation of human macrophages. Comparison of other cytokines with interferon-gamma. J Exp Med. 1984 Aug 1;160(2):600–605. doi: 10.1084/jem.160.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Guyre P. M. Inhibition of growth of Toxoplasma gondii in cultured fibroblasts by human recombinant gamma interferon. Infect Immun. 1984 May;44(2):211–216. doi: 10.1128/iai.44.2.211-216.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray C. G. The ontogeny of interferon production by human leukocytes. J Pediatr. 1970 Jan;76(1):94–98. doi: 10.1016/s0022-3476(70)80136-6. [DOI] [PubMed] [Google Scholar]
- Remington J. S., Merigan T. C. Interferon: protection of cells infected with an intracellular protozoan (Toxoplasma gondii). Science. 1968 Aug 23;161(3843):804–806. doi: 10.1126/science.161.3843.804. [DOI] [PubMed] [Google Scholar]
- Rinderknecht E., O'Connor B. H., Rodriguez H. Natural human interferon-gamma. Complete amino acid sequence and determination of sites of glycosylation. J Biol Chem. 1984 Jun 10;259(11):6790–6797. [PubMed] [Google Scholar]
- Schreiber R. D., Pace J. L., Russell S. W., Altman A., Katz D. H. Macrophage-activating factor produced by a T cell hybridoma: physiochemical and biosynthetic resemblance to gamma-interferon. J Immunol. 1983 Aug;131(2):826–832. [PubMed] [Google Scholar]
- Shirahata T., Shimizu K. Some physicochemical characteristics of an immune lymphocyte product which inhibits the multiplication of toxoplasma within mouse macrophages. Microbiol Immunol. 1979;23(1):17–30. doi: 10.1111/j.1348-0421.1979.tb00436.x. [DOI] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Inhibition of the growth of Rickettsia prowazekii in cultured fibroblasts by lymphokines. J Exp Med. 1983 Mar 1;157(3):974–986. doi: 10.1084/jem.157.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakasugi N., Virelizier J. L. Defective IFN-gamma production in the human neonate. I. Dysregulation rather than intrinsic abnormality. J Immunol. 1985 Jan;134(1):167–171. [PubMed] [Google Scholar]
- Wilson C. B., Haas J. E. Cellular defenses against Toxoplasma gondii in newborns. J Clin Invest. 1984 Jun;73(6):1606–1616. doi: 10.1172/JCI111367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. B., Haas J. E., Weaver W. M. Isolation, purification and characteristics of mononuclear phagocytes from human placentas. J Immunol Methods. 1983 Feb 11;56(3):305–317. doi: 10.1016/s0022-1759(83)80020-9. [DOI] [PubMed] [Google Scholar]
- Wilson C. B., Remington J. S. Activity of human blood leukocytes against Toxoplasma gondii. J Infect Dis. 1979 Dec;140(6):890–895. doi: 10.1093/infdis/140.6.890. [DOI] [PubMed] [Google Scholar]
- Wilson C. B., Remington J. S. Effects of monocytes from human neonates on lymphocyte transformation. Clin Exp Immunol. 1979 Jun;36(3):511–520. [PMC free article] [PubMed] [Google Scholar]
- Wilson C. B., Tsai V., Remington J. S. Failure to trigger the oxidative metabolic burst by normal macrophages: possible mechanism for survival of intracellular pathogens. J Exp Med. 1980 Feb 1;151(2):328–346. doi: 10.1084/jem.151.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Waddell A. Interferonlike factors from antigen- and mitogen-stimulated human leukocytes with antirickettsial and cytolytic actions on Rickettsia prowazekii. Infected human endothelial cells, fibroblasts, and macrophages. J Exp Med. 1983 Jun 1;157(6):1780–1793. doi: 10.1084/jem.157.6.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]


