Abstract
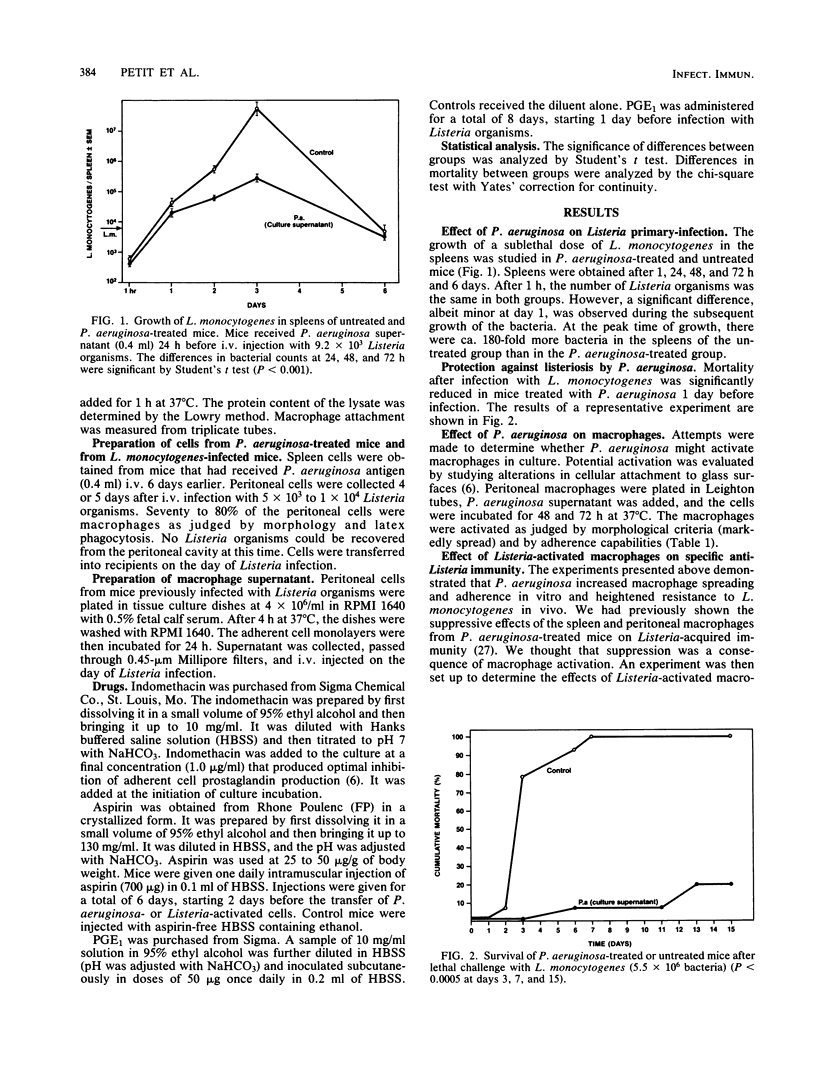
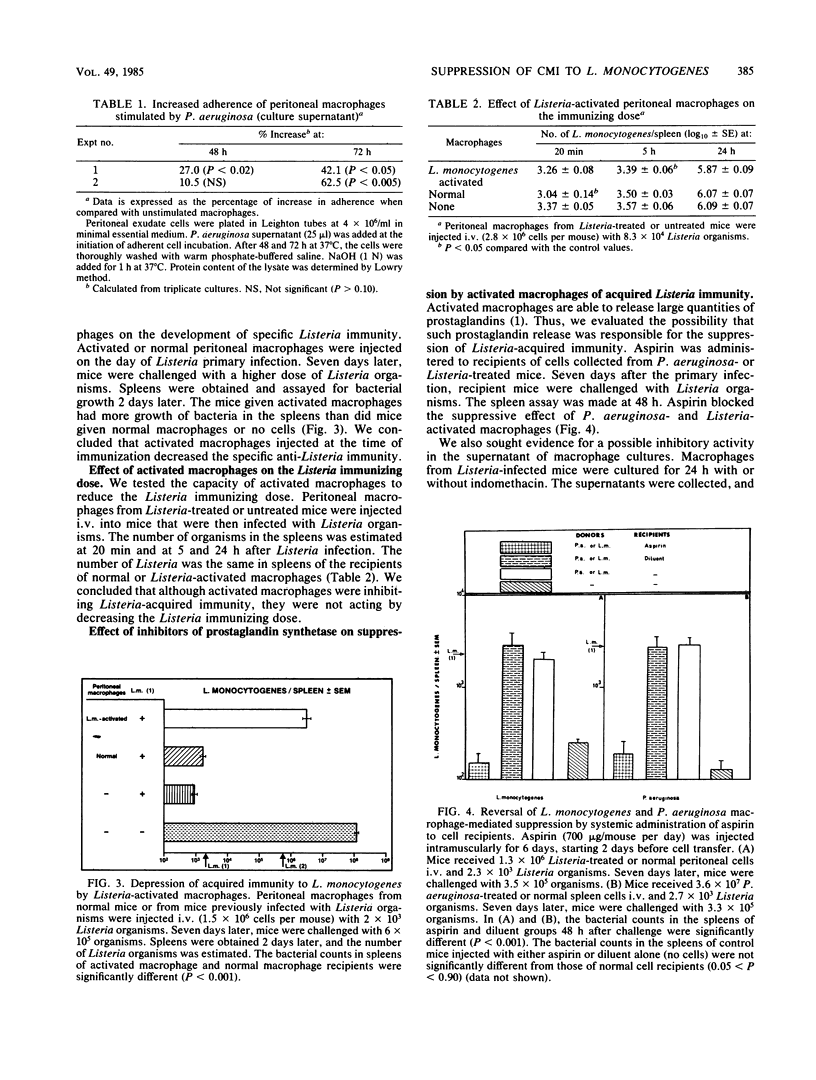
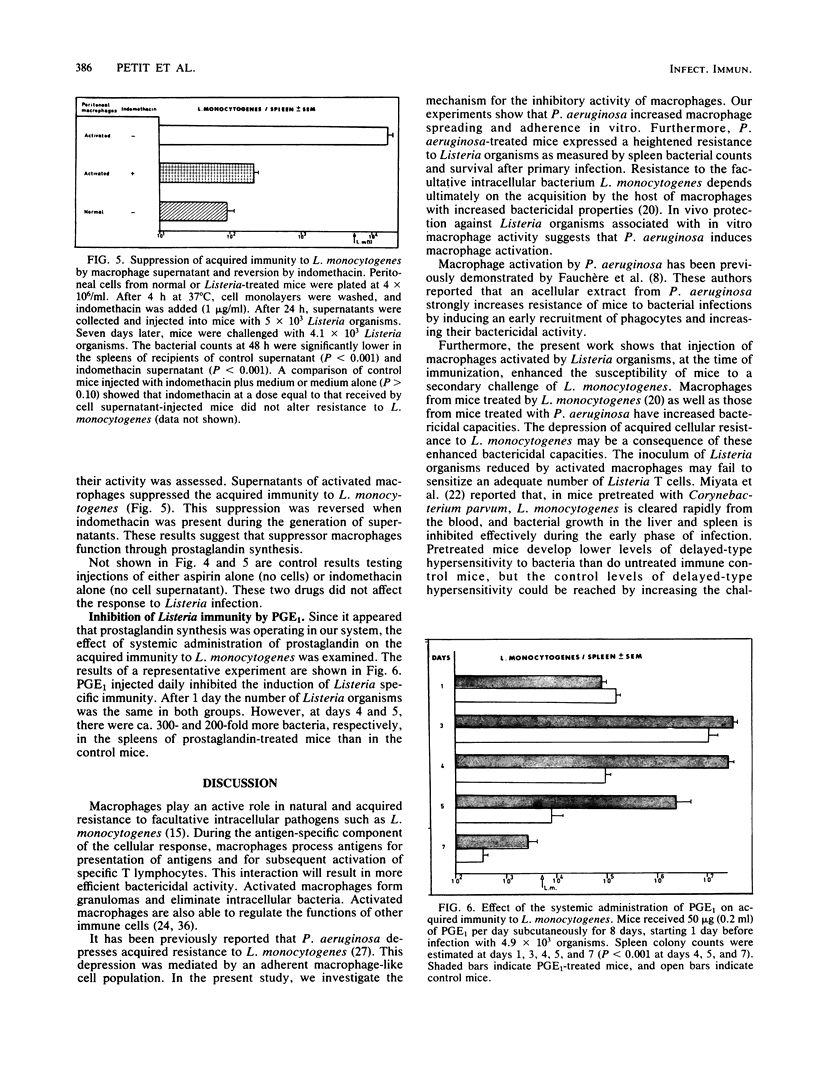
We previously demonstrated the suppression of cell-mediated immunity to Listeria monocytogenes by Pseudomonas aeruginosa-induced, macrophage-like cells. The present study was undertaken to evaluate the mechanism for this suppression. P. aeruginosa supernatant was shown to activate macrophages by the criteria of increased bactericidal capacities and increased attachment to glass surfaces. Acquired cellular resistance to L. monocytogenes could also be inhibited by macrophages from L. monocytogenes-pretreated mice. The depression of acquired immunity by P. aeruginosa- or L. monocytogenes-activated macrophages did not appear to be due to a reduction of antigenic stimulus after nonspecific macrophage activation. In contrast, our findings suggest that suppression is mediated by activated macrophages through a prostaglandin-dependent mechanism. In vivo administration of aspirin blocked the immunosuppressive effect of P. aeruginosa- or L. monocytogenes-activated cells. Moreover, the suppressive activity of supernatants of macrophages from Listeria-infected mice was reversed when indomethacin was present during supernatant generation. Finally, prostaglandin E1 treatment in vivo profoundly inhibited the induction of cell-mediated immunity to L. monocytogenes. The possible role and mechanism of prostaglandin in suppressing cellular immunity to intracellular bacteria are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. Mechanisms by which activated macrophages inhibit lymphocyte responses. Immunol Rev. 1978;40:3–27. doi: 10.1111/j.1600-065x.1978.tb00399.x. [DOI] [PubMed] [Google Scholar]
- Cahill J., Hopper K. E. Immunoregulation by macrophages: differential secretion of prostaglandin E and interleukin 1 during infection with Salmonella enteritidis. Cell Immunol. 1982 Mar 1;67(2):229–240. doi: 10.1016/0008-8749(82)90216-7. [DOI] [PubMed] [Google Scholar]
- Ellner J. J., Spagnuolo P. J. Suppression of antigen and mitogen induced human T lymphocyte DNA synthesis by bacterial lipopolysaccharide: mediation by monocyte activation and production of prostaglandins. J Immunol. 1979 Dec;123(6):2689–2695. [PubMed] [Google Scholar]
- Fauchère J. L., simonet M., Véron M. Non-specific resistance of mice to bacteria, induced by an acellular extract from Pseudomonas aeruginosa. Ann Immunol (Paris) 1982 May-Jun;133C(3):333–347. doi: 10.1016/0769-2625(82)90046-0. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Messner R. P., Peake G. T. Prostaglandin suppression of mitogen-stimulated lymphocytes in vitro. Changes with mitogen dose and preincubation. J Clin Invest. 1978 Oct;62(4):753–760. doi: 10.1172/JCI109186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin J. S., Webb D. R. Regulation of the immune response by prostaglandins. Clin Immunol Immunopathol. 1980 Jan;15(1):106–122. doi: 10.1016/0090-1229(80)90024-0. [DOI] [PubMed] [Google Scholar]
- Gordon D., Bray M. A., Morley J. Control of lymphokine secretion by prostaglandins. Nature. 1976 Jul 29;262(5567):401–402. doi: 10.1038/262401a0. [DOI] [PubMed] [Google Scholar]
- Grimm W., Seitz M., Kirchner H., Gemsa D. Prostaglandin synthesis in spleen cell cultures of mice injected with Corynebacterium parvum. Cell Immunol. 1978 Oct;40(2):419–426. doi: 10.1016/0008-8749(78)90349-0. [DOI] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Kato K., Yamamoto K. Involvement of prostaglandin E1 in delayed-type hypersensitivity suppression induced with live Mycobacterium bovis BCG. Infect Immun. 1982 Apr;36(1):426–429. doi: 10.1128/iai.36.1.426-429.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Simon M. M., Hahn H. Regulatory interactions between macrophages and T-cell subsets in Listeria monocytogenes-specific T-cell activation. Infect Immun. 1982 Dec;38(3):907–913. doi: 10.1128/iai.38.3.907-913.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel S. L., Chensue S. W., Plewa M., Higashi G. I. Macrophage function in the Schistosoma mansoni egg-induced pulmonary granuloma. Role of arachidonic acid metabolites in macrophage Ia antigen expression. Am J Pathol. 1984 Feb;114(2):240–249. [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miake S., Nomoto K., Matsumoto T., Yoshikai Y., Takeya K. Peritoneal exudation of macrophages by irritants and its effect on immune responses against sheep erythrocytes and Listeria monocytogenes. Jpn J Exp Med. 1983 Apr;53(2):95–102. [PubMed] [Google Scholar]
- Miyata H., Nomoto K., Takeya K. Characteristics of resistance to Listeria monocytogenes enhanced by Corynebacterium parvum in mice. Immunology. 1980 May;40(1):33–39. [PMC free article] [PubMed] [Google Scholar]
- Novogrodsky A., Rubin A. L., Stenzel K. H. Selective suppression by adherent cells, prostaglandin, and cyclic AMP analogues of blastogenesis induced by different mitogens. J Immunol. 1979 Jan;122(1):1–7. [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Immune response to atypical mycobacteria: immunocompetence of heavily infected mice measured in vivo fails to substantiate immunosuppression data obtained in vitro. Infect Immun. 1984 Jan;43(1):32–37. doi: 10.1128/iai.43.1.32-37.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit J. C., Daguet G. L. Interference of Pseudomonas aeruginosa with immunospecific host defenses. Biomed Pharmacother. 1983;37(9-10):422–428. [PubMed] [Google Scholar]
- Petit J. C., Richard G., Albert B., Daguet G. L. Depression by Pseudomonas aeruginosa of two T-cell-mediated responses, anti-Listeria immunity and delayed-type hypersensitivity to sheep erythrocytes. Infect Immun. 1982 Mar;35(3):900–908. doi: 10.1128/iai.35.3.900-908.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit J. C., Unanue E. R. Effects of bacterial products on lymphocytes and macrophages: their possible role in natural resistance to listeria infetion in mice. J Immunol. 1974 Sep;113(3):984–992. [PubMed] [Google Scholar]
- Rouzer C. A., Scott W. A., Hamill A. L., Liu F. T., Katz D. H., Cohn Z. A. Secretion of leukotriene C and other arachidonic acid metabolites by macrophages challenged with immunoglobulin E immune complexes. J Exp Med. 1982 Oct 1;156(4):1077–1086. doi: 10.1084/jem.156.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab J. H. Suppression of the immune response by microorganisms. Bacteriol Rev. 1975 Jun;39(2):121–143. doi: 10.1128/br.39.2.121-143.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder D. S., Beller D. I., Unanue E. R. Prostaglandins modulate macrophage Ia expression. Nature. 1982 Sep 9;299(5879):163–165. doi: 10.1038/299163a0. [DOI] [PubMed] [Google Scholar]
- Stenson W. F., Parker C. W. Prostaglandins, macrophages, and immunity. J Immunol. 1980 Jul;125(1):1–5. [PubMed] [Google Scholar]
- Tweardy D. J., Schacter B. Z., Ellner J. J. Association of altered dynamics of monocyte surface expression of human leukocyte antigen DR with immunosuppression in tuberculosis. J Infect Dis. 1984 Jan;149(1):31–37. doi: 10.1093/infdis/149.1.31. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. The regulation of lymphocyte functions by the macrophage. Immunol Rev. 1978;40:227–255. doi: 10.1111/j.1600-065x.1978.tb00408.x. [DOI] [PubMed] [Google Scholar]
- Virgin H. W., 4th, Unanue E. R. Suppression of the immune response to Listeria monocytogenes. I. Immune complexes inhibit resistance. J Immunol. 1984 Jul;133(1):104–109. [PubMed] [Google Scholar]
- Watson S. R., Bullock W. E. Immunoregulatory defects in leprosy. Adv Exp Med Biol. 1983;162:203–215. doi: 10.1007/978-1-4684-4481-0_20. [DOI] [PubMed] [Google Scholar]
- Wren S. M., Wepsic H. T., Larson C. H., De Silva M. A., Mizushima Y. Inhibition of the graft-versus-host response by BCGcw-induced suppressor cells or prostaglandin E1. Cell Immunol. 1983 Mar;76(2):361–371. doi: 10.1016/0008-8749(83)90379-9. [DOI] [PubMed] [Google Scholar]


