Abstract
Pre-B-cell growth-stimulating factor/stromal cell-derived factor 1 (PBSF/SDF-1) is a member of the CXC group of chemokines that is initially identified as a bone marrow stromal cell-derived factor and as a pre-B-cell stimulatory factor. Although most chemokines are thought to be inducible inflammatory mediators, PBSF/SDF-1 is essential for perinatal viability, B lymphopoiesis, bone marrow myelopoiesis, and cardiac ventricular septal formation, and it has chemotactic activities on resting lymphocytes and monocytes. In this paper, we have isolated a cDNA that encodes a seven transmembrane-spanning-domain receptor, designated pre-B-cell-derived chemokine receptor (PB-CKR) from a murine pre-B-cell clone, DW34. The deduced amino acid sequence has 90% identity with that of a HUMSTSR/fusin, a human immunodeficiency virus 1 (HIV-1) entry coreceptor. However, the second extracellular region has lower identity (67%) compared with HUMSTSR/fusin. PB-CKR is expressed during embryo genesis and in many organs and T cells of adult mice. Murine PBSF/SDF-1 induced an increase in intracellular free Ca2+ in DW34 cells and PB-CKR-transfected Chinese hamster ovary (CHO) cells, suggesting that PB-CKR is a functional receptor for murine PBSF/SDF-1. Murine PBSF/SDF-1 also induced Ca2+ influx in fusin-transfected CHO cells. On the other hand, considering previous results that HIV-1 does not enter murine T cells that expressed human CD4, PB-CKR may not support HIV-1 infection. Thus, PB-CKR will be an important tool for functional mapping of HIV-1 entry coreceptor fusin and for understanding the function of PBSF/SDF-1 further.
Keywords: chemokine, B lymphopoiesis, bone marrow, myelopoiesis, cardiogenesis
Pre-B-cell growth-stimulating factor/stromal cell-derived factor 1 (PBSF/SDF-1) is a member of the CXC group of chemokines that is initially identified as a bone marrow stromal cell-derived solule factor and as a bone marrow stromal cell-derived pre-B-cell stimulatory factor (1, 2). The chemokines are a large family of small structurally related cytokines that have four conserved cysteines forming two disulfide bonds (3, 4). There are two subgroups according to the arrangement of the first two cysteines that are either adjacent (CC subfamily) or separated by one amino acid (CXC subfamily). The CC subfamily includes monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory peptide-1α (MIP-1α), RANTES, and eotaxin. The CXC subfamily includes interleukin (IL) 8, platelet factor-4 (PF4), γ-interferon-inducible protein (IP-10), and PBSF/SDF-1.
The evolutionary and sequence homology properties of PBSF/SDF-1 are unique among chemokines. Although PBSF/SDF-1 belongs to the CXC subfamily, it is almost equally related to both CC and CXC chemokines, based on amino acid sequence homologies (5). Conservation of amino acid sequences between mouse and human is strong in comparison with other chemokines (6).
Consistent with these findings, PBSF/SDF-1 has functions that are unique among members of the chemokine family. Although most chemokines are thought to be inducible inflammatory mediators that determine leukocyte chemotaxis, PBSF/SDF-1 is essential for perinatal viability, B lymphopoiesis, bone marrow myelopoiesis, and cardiac ventricular septal formation (7). Recently, PBSF/SDF-1 was shown to have chemotactic activities on resting T lymphocytes and monocytes, suggesting that it has a function in immune surveillance (5). Transcripts of PBSF/SDF-1 are constitutively expressed in many organs, including brain, heart, lung, liver, thymus, spleen, and kidney (1). The functions of PBSF/SDF-1 in each organ have yet to be elucidated.
To understand the function of PBSF/SDF-1 further, we isolated a cDNA encoding a murine PBSF/SDF-1 receptor from the PBSF/SDF-1-responsive pre-B-cell clone DW34 and found that it is closely related to the IL-8 receptors and has 90% identity with a previously identified receptor HUMSTSR/HM89/LESTR/fusin (8–10), a human immunodeficiency virus 1 (HIV-1) entry coreceptor (11).
METHODS
Materials.
SDF-1α was purified from supernatants of the murine bone marrow stromal cell line MS-5 (5). Dw34 cells were provided by S.-I. Nishikawa (Kyoto University Faculty of Medicine, Kyoto). A λgt10 cDNA library was provided by K. Morishita (National Cancer Center Research Institute, Tokyo). An expression vector pCAGGS was provided by J. Miyazaki (Osaka University Medical School, Osaka).
PCR of pre-B-cell-Derived Chemokine Receptor (PB-CKR), Subcloning, and Sequencing.
Total RNA (1–2 μg) from DW34 cells were used as substrate in a reverse transcription-coupled PCR. Degenerate oligonucleotides corresponding to conserved regions of chemoattractant receptors were used in the PCR. PCR conditions were 40 cycles at 92°C for 1.0 min, 53°C for 1.0 min, and 72°C for 2.0 min. PCR products were cloned into the pT7Blue and expression vector pCAGGSneo.
5′-End Amplification of cDNAs by Rapid Amplification of cDNA Ends (5′-RACE).
5′-RACE was performed with the Marathon cDNA amplification kit (CLONTECH). We produced double-stranded cDNA from DW34 mRNA purified as described (2) and ligated to the Marathon cDNA adaptor. 5′-RACE PCR products were primed with an internal gene-specific primer (TCATCCCCCTGACTGATGTCCCCCT) and the Marathon adaptor primer AP-1. Amplified DNA was isolated, cloned, and sequenced.
DNA Transfections.
Chinese hamster ovary (CHO) cells were transfected with 10–20 μg of plasmid DNA by the modified calcium phosphate method (12) and G418-resistant cells were picked and expanded.
Intracellular Ca2+ Measurements.
Cells were suspended in HBSS buffer (20 mM Hepes/125 mM NaCl/1 mM KCl/0.5 mM glucose/0.025% bovine serum albumin/1 mM CaCl2/1 mM MgCl2) and loaded with 2.5 μM fura-PE3 per 106 cells in 0.5 ml of HBSS buffer for 30 min at 37°C. Intracellular Ca2+ measurements were made on the Perkin–Elmer LS 50B spectrofluorometer as described (13).
Screening cDNA Libraries.
A λgt10 cDNA library made from NFS58 was screened with 32P-labeled cloned PCR products of PB-CKR (TM2-TM7; TM is transmembrane domain) described below. Phage DNA was isolated and subcloned into pBluescript.
Northern and Southern Blot Hybridization.
Total RNA was isolated using the ISOGEN method as recommended by the supplier (Nippon Gene, Tokyo). Poly(A)+ RNA was isolated using Dynabeads oligo(dT) (Dynal, Great Neck, NY). RNA was fractionated on formaldehyde gels, blotted onto a nylon membrane, and hybridized as described. A Southern blot filter containing human, monkey, rat, mouse, dog, cow, rabbit, and chicken was used for hybridization with the PB-CKR probe. The filter was washed with 2× standard saline citrate (SSC)/0.05% SDS at 65°C for 40 min and autoradiographed.
RESULTS
Molecular Cloning and Structure of a Murine PBSF/SDF-1 Receptor.
All known chemokine receptors are single-chain G-protein-coupled seven transmembrane receptors and amino acid sequences are conserved among them. On the basis of the structural similarities between members of this group of cytokines, it was likely that the corresponding receptors for PBSF/SDF-1 would be of the seven-transmembrane-segment class. Accordingly, we synthesized four degenerate oligonucleotides corresponding to conserved amino acid sequences in transmembrane regions of the previously identified chemoattractant receptors, IL-8RA, IL-8RB, MCP-1R, hC5a, and HUMSTSR. The first oligonucleotide corresponded to a region in TM1 (TM1p), (L/F)LP(P/T)(L/I)YS, the second to a region in TM2 (TM2p), L(N/H)L(A/S)(I/V)(S/A)DLLF, the third to a region in TM3 (TM3p), YLAIVHA, and the forth to a region in TM7 (TM7p), HCC(V/L/I)NP(V/L/I)(I/L)Y. These were then used as primers in reverse transcription-coupled PCR experiments using cDNA substrates from a PBSF/SDF-1 responsive pre-B-cell clone, DW34. Four pairs of primers (TM1p-TM3p, TM1p-TM4p, TM2p-TM3p, and TM2p-TM4p) were employed in the PCR experiments. This approach yielded four major amplified DNA bands ranging in size from 350 to 750 bp. The entire PCR-amplified DNA was then cloned into pT7Blue. Interestingly, sequencing of the inserts revealed that all clones were fragments of the same novel seven-transmembrane-domain receptor, tentatively named PB-CKR. The 5′ end of the cDNA corresponding to the clones was isolated using 5′ RACE. The 3′ end of the PB-CKR cDNA was isolated by screening a λgt10 cDNA library made from NFS58 cells. The first oligonucleotide corresponded to a 5′ end of the cDNA and the second to a 3′ end of the cDNA were used as primers in PCR from cDNA substrates constructed from DW34 cells. This approach yeilded a 1077-bp single-amplified DNA band. Sequence analysis revealed that the amplified DNA included the entire open reading frame of PB-CKR that contains seven putative membrane-spanning domains, which are typical of G protein-coupled receptors, separating four extracellular and four intracellular regions. To our surprise, PB-CKR shares 90% amino acid identity with previously identified human receptor HUMSTSR/HM89/LESTR/fusin (8–10), a HIV-1 entry coreceptor (11). Interestingly, lower sequence identity (67%) with fusin is found in the second extracellular loop region (Fig. 1). There is an insertion of 7 amino acids in the region (Q181-G187) compared with HUMSTSR/HM89/LESTR/fusin. The N-terminal region and the third extracellular loop region that are shown to be important for ligand binding of other chemokine receptors have 79% amino acid identity with HUMSTSR/HM89/LESTR/fusin.
Figure 1.
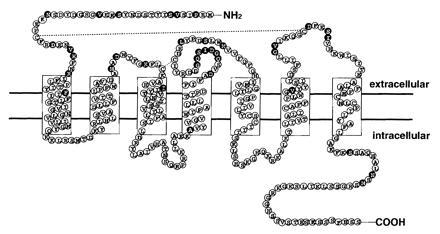
Deduced amino acid sequence and transmembrane model of PB-CKR. The transmembrane-spanning domains were identified by hydropathy analysis and homology to other G-protein-coupled receptors. The solid circles represent amino acid substitutions of fusin.
Signaling Through the Murine PBSF/SDF-1 Receptor in Response to Murine PBSF/SDF-1.
Murine PBSF/SDF-1 stimulates the proliferation or survival of DW34 cells with or without IL-7 (2). Since a ligand-stimulated increase in Ca2+ was obtained in responsive cells with most of the chemokines, we examined the ability of murine PBSF/SDF-1 to induce an increase in intracellular Ca2+ in DW34 cells. Murine PBSF/SDF-1 applied at 100 nM induced prolonged increase in cytosolic free Ca2+ concentration ([Ca2+]i) that peaked at 50–60 s (Fig. 2A). To determine whether DW34-derived PB-CKR is a functional murine PBSF/SDF-1 receptor, PB-CKR was expressed in CHO cells and intracellular Ca2+ levels in response to murine PBSF/SDF-1 were measured. When PB-CKR-transfected cells loaded with fura-PE3 were challenged with 100 nM murine PBSF/SDF-1, a rapid increase in intracellular Ca2+ that peaked at 10–20 s was observed (Fig. 2B). Murine PBSF/SDF-1 blocked the response to a second challenge, indicating that complete desensitization has occurred (Fig. 2E). Control cells transfected with vector alone (not shown) or with vector containing human MCP-1 receptor (Fig. 2C) did not respond to the murine PBSF/SDF-1. The control cells transfected with human MCP-1 receptor responded to human MCP-1 (Fig. 2C). Since amino acid sequences of fusin has 90% amino acid identity with PB-CKR, CHO cells transfected with fusin were tested. Murine PBSF/SDF-1 also induced Ca2+ flux through fusin (Fig. 2D).
Figure 2.
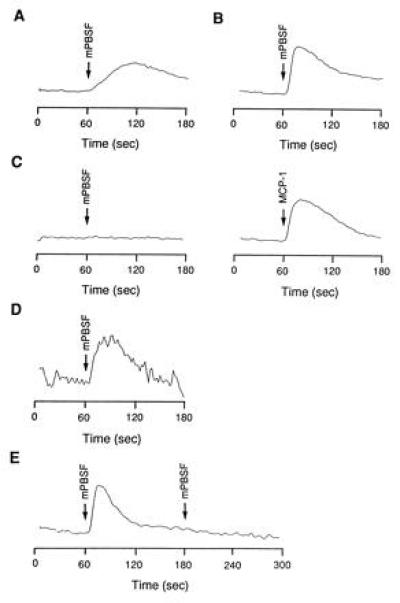
Intracellular Ca2+ response of DW34 cells and CHO cells transfected with PB-CKR in response to murine PBSF/SDF-1. Ligands were added at the time indicated by the arrow. Rise in [Ca2+]i is represented by the increase in relative fluorescence. DW34 cells (A) and CHO cells transfected with a vector encoding the PB-CKR (B and E) and fusin (D) were challenged with murine PBSF/SDF-1 once (A, B, and D) or twice (E). CHO cells transfected with a vector encoding the human MCP-1 receptor were challenged with murine PBSF/SDF-1 and human MCP-1 (C).
Expression and Evolutionary Conservation of Murine PBSF/SDF-1 Receptor.
Expression of PB-CKR was examined with Northern blots. PB-CKR mRNA was expressed in embryos at embryonic day 7.5, 11.5, 15.5, and 17.5 and in brain, thymus, lymph node, spleen, stomach, small intestine, and kidney of adult mice (Fig. 3). The absence of expression in liver was observed (Fig. 3). PB-CKR mRNA was much more abundant in thymus, spleen, and lymph node compared with other organs. Since PB-CKR is a murine counterpart of fusin that is essential for infection of T-cell-line-tropic HIV-1 into T cells, we also examined the expression of PB-CKR in murine T cells. PB-CKR mRNA was expressed in both double-positive (CD4+CD8+) and single-positive (CD4+CD8−, CD4−CD8+) thymocytes (data not shown). Southern blot analysis under low-stringency hybridization and washing condition showed that PB-CKR gene is conserved in human, monkey, rat, mouse, dog, cow, and chicken genomic DNAs (Fig. 4).
Figure 3.
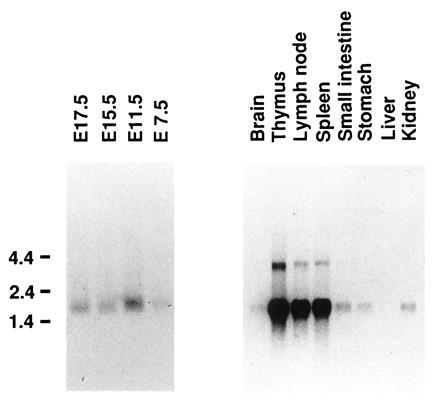
Northern blot analysis of PB-CKR mRNA expression. Poly(A)+ RNA from murine tissues was size-fractionated on formaldehyde/agarose gel, transferred to a nylon filter, and hybridized with a radiolabeled fragment of PB-CKR cDNA. Sizes (kb) of relevant marker bands are shown at left.
Figure 4.
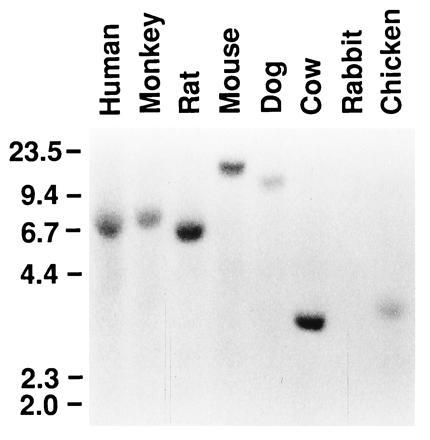
Conservation of the PB-CKR gene in various species. A Southern blot filter that contained EcoRI-digested genomic DNA from human, monkey, rat, mouse, dog, cow, rabbit, and chicken was hybridized with a DNA probe from PB-CKR. Sizes (kb) of relevant marker bands are shown at left.
DISCUSSION
In this study, we isolated a murine PBSF/SDF-1 receptor, PB-CKR, and demonstrated that it is a murine counterpart of fusin, a HIV-1 entry coreceptor. While this manuscript was in preparation, human PBSF/SDF-1 was shown to be a ligand for fusin, designated CXCR4, and to block HIV-1 entry into T cells (14, 15). Therefore, we designate PB-CKR as murine CXCR4/fusin. Recently, two HIV-1 entry coreceptors, fusin and CC-CKR-5, were discovered and shown to be important in infection of HIV-1 (16–22). Fusin is required for entry of T-cell-line-tropic HIV-1 virions into human CD4-expressing cells (11). Our results showed murine CXCR4/fusin mRNA was expressed in murine T cells. However, murine T cells expressing the human CD4 were refractory to HIV-1 infection in vitro and in vivo (23, 24). These results indicate that human CXCR4/fusin enables human CD4-expressing cells to support HIV-1 infection but its murine counterpart does not. On the other hand, we have shown that both fusin and its murine counterpart were responsive to murine PBSF/SDF-1. Considering high conservation of amino acid sequences between murine and human fusin (Fig. 1), amino acid substitutions in the second extracellular loop of fusin that has lower identity with its murine counterpart may be critical for HIV-1 entry but not for ligand binding. Studying mouse–human chimeric receptors may identify the amino acid residues of fusin that are directly involved in the entry of HIV-1 into T cells.
Murine PBSF/SDF-1 elicited a prolonged increase in [Ca2+]i in pre-B-cell DW34 cells (Fig. 2A), in contrast to an earlier and more transient increase in [Ca2+]i in CHO cells transfected with murine CXCR4/fusin (Fig. 2B). Most other chemokines have been shown to induce latter type of responses in their target cells. Since PBSF/SDF-1 has multiple functions and acts on a variety of target cells, it may induce prolonged responses in some target cells and earlier and more transient responses in others. It would be interesting to determine whether the PBSF/SDF-1-induced response observed in DW34 cells is mediated through murine CXCR4/fusin and links to G proteins.
Murine CXCR4/fusin is expressed during embryogenesis and in many organs of adult mice. Amino acid sequence of CXCR4 is highly conserved (90%) between mouse and human, compared with other CXC chemokine receptors, IL-8 receptor A (68%) or B (71%). These results are mirrored in its ligand PBSF/SDF-1 and support the idea that murine CXCR4 is a physiological receptor for murine PBSF/SDF-1. However, it is important to investigate the physiological functions of murine CXCR4 and determine whether there are other receptors for murine PBSF/SDF-1.
Acknowledgments
We thank Dr. S.-I. Nishikawa, Dr. K. Morishita, R. Yoshida, and Dr. J. Miyazaki for valuable reagents; F. Ueno, and Y. Kataoka for technical assistance; K. Kubota and K. Kawamori for secretarial help. This work was supported by grants from the Ministry of Education, Science, and Culture of Japan.
Footnotes
Abbreviations: PBSF, pre-B-cell growth-stimulating factor; SDF-1, stromal cell-derived factor 1; [Ca2+]i, cytosolic free Ca2+ concentration; HIV-1, human immunodeficiency virus 1; IL, interleukin; TM, transmembrane domain; RACE, rapid amplification of cDNA ends; PB-CKR, pre-B-cell-derived chemokine receptor.
Data deposition: The sequence reported in this paper has been deposited in the GenBank data base (accession no. D87747D87747).
References
- 1.Tashiro K, Tada H, Heilker R, Shirozu M, Nakano T, Honjo T. Science. 1993;261:600–603. doi: 10.1126/science.8342023. [DOI] [PubMed] [Google Scholar]
- 2.Nagasawa T, Kikutani H, Kishimoto T. Proc Natl Acad Sci USA. 1994;91:2305–2309. doi: 10.1073/pnas.91.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oppenheim J J, Zachariae O C, Mukaida N, Matsushima K. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- 4.Baggiolini M, Dewald B, Moser B. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 5.Bleul C C, Fuhlbrigge R C, Casasnovas J M, Aiuti A, Springer T A. J Exp Med. 1996;184:1101–1110. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shirozu M, Nakano T, Inazawa J, Tashiro K, Tada H, Shinohara T, Honjo T. Genomics. 1995;28:495–500. doi: 10.1006/geno.1995.1180. [DOI] [PubMed] [Google Scholar]
- 7.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S-I, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Nature (London) 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 8.Federsppiel B, Mekhado I G, Duncan A M V, Delaney A, Schappert K, Clark-Lewis I, Jirik F R. Genomics. 1993;16:707–712. doi: 10.1006/geno.1993.1251. [DOI] [PubMed] [Google Scholar]
- 9.Nomura H, Nielsen B W, Matsushima K. Int Immunol. 1993;5:1239–1249. doi: 10.1093/intimm/5.10.1239. [DOI] [PubMed] [Google Scholar]
- 10.Loetscher M, Geiser T, O, Reilly T, Zwahlen R, Baggiolini M, Moser B. J Biol Chem. 1994;269:232–237. [PubMed] [Google Scholar]
- 11.Feng Y, Broder C C, Kennedy P E, Berger E A. Science. 1996;272:872–877. [Google Scholar]
- 12.Chen C, Okayama H. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitaura M, Nakajima T, Imai T, Harada S, Combadiere C, Tiffany H L, Murphy P M, Yoshie O. J Biol Chem. 1996;271:7725–7730. doi: 10.1074/jbc.271.13.7725. [DOI] [PubMed] [Google Scholar]
- 14.Bleul C C, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer T A. Nature (London) 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 15.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier J-L, Arenzana-Seisdedos F, Schwartz O, Heard J-M, Clark-Lewis I, Legler D F, Loetscher M, Baggiolini M, Moser B. Nature (London) 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 16.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Marzio P D, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Shall T J, Littman D R, Landau N R. Nature (London) 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 17.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. Nature (London) 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 18.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 19.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 20.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 21.Liu R, Paxton W A, Choe S, Ceradini D, Martin S R, Horuk R, MacDonald M E, Stuhlmann H, Koup R A, Landau N R. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 22.Samson M, Libert F, Doranz B J, Rucker J, Liesnard C, Farber C-M, Saragosti S, Lapoumeroulie C, Cognaux J, Forceille C, Muyldermans G, Verhofstede C, Burtonboy G, Georges M, Imai T, Rana S, Yi Y, Smyth R J, Collman R G, Doms R W, Vassart G, Parmentier M. Nature (London) 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 23.Maddon P J, Dalgleish A G, McDougal J S, Clapham P R, Weiss R A, Axel R. Cell. 1986;47:333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- 24.Lores P, Boucher V, Mackay C, Pla M, Von Boehmer H, Jami J, Barre-Sinoussi F, Weill J-C. AIDS Res Hum Retroviruses. 1992;8:2063–2071. doi: 10.1089/aid.1992.8.2063. [DOI] [PubMed] [Google Scholar]


