Abstract
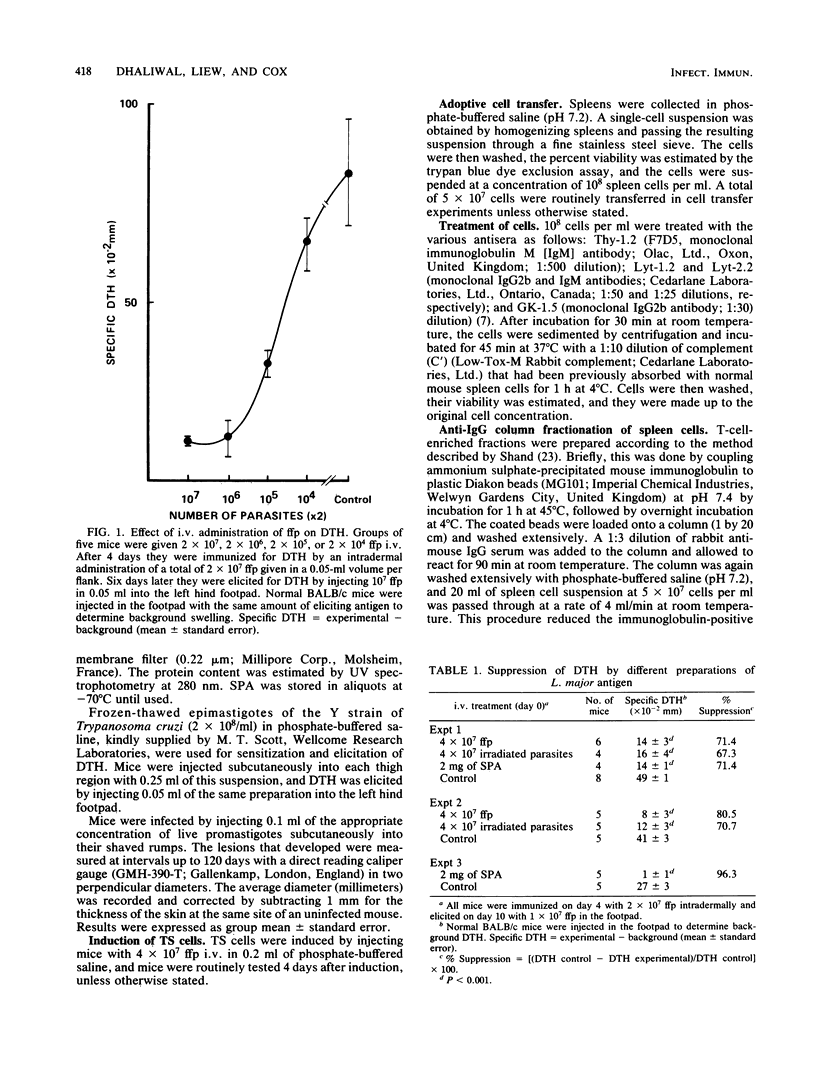
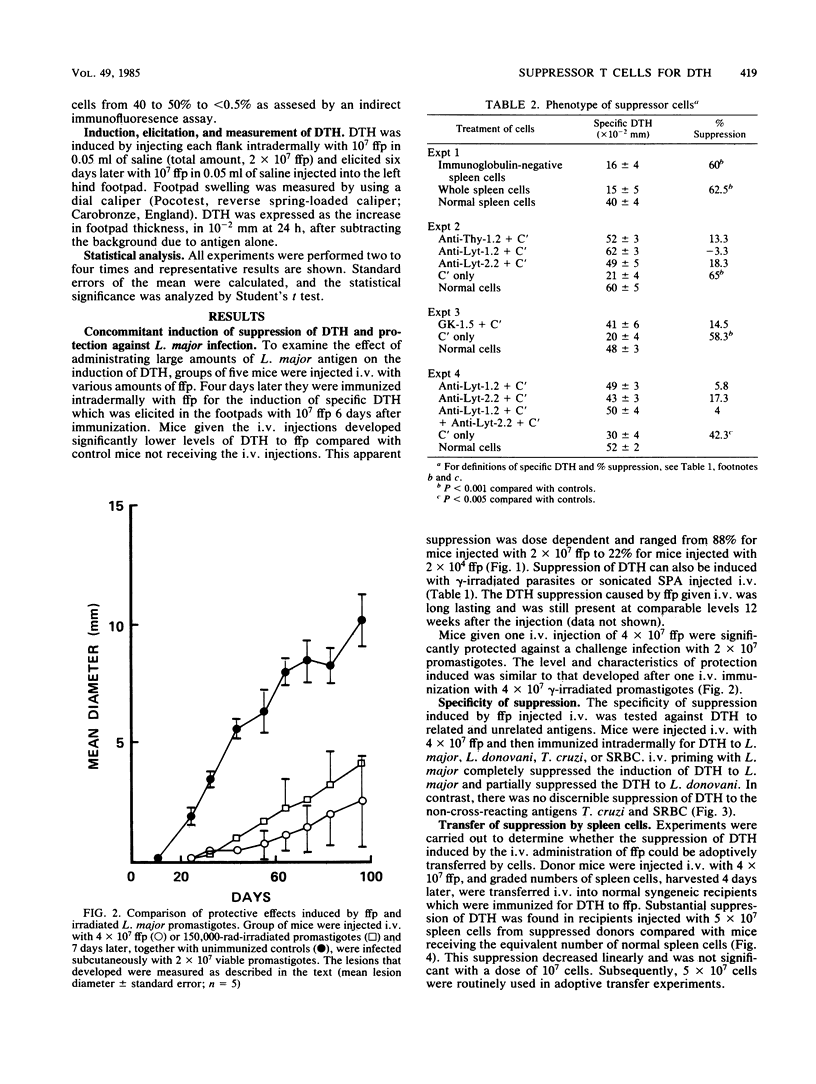
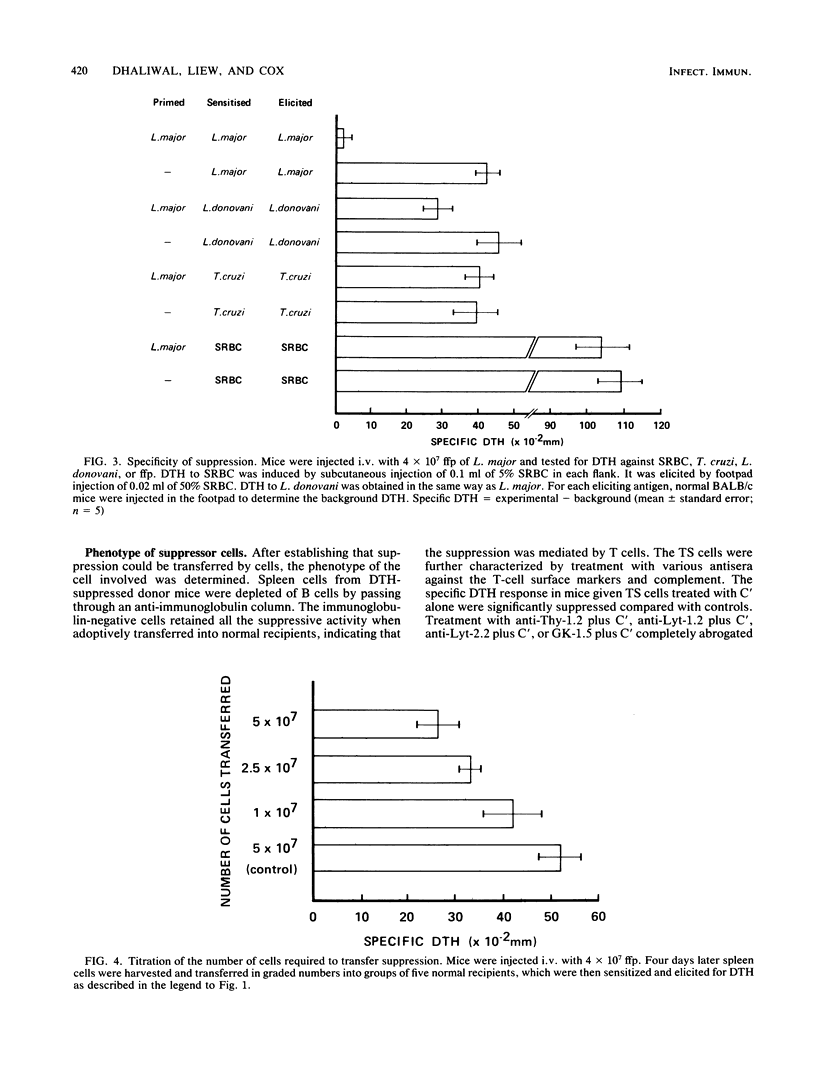
BALB/c mice injected intravenously with 10(6) or higher doses of formaldehyde-fixed promastigotes (ffp) of Leishmania major developed significantly lower levels of delayed-type hypersensitivity (DTH) compared with uninjected control mice when they were subsequently immunized intradermally with ffp. The suppression of DTH was antigen specific and was also inducible with lethally irradiated promastigotes or soluble parasite antigens. The suppressive effect was adoptively transferable with splenic T cells which express the Lyt-1+2+ and L3T4+ phenotypes. These specific suppressor T cells were active against both the inductive and expressive phases of DTH. They were sensitive to 200 rads of gamma-irradiation in vitro and appeared to manifest the suppressive activity via soluble factors. In spite of this profound suppression of DTH, BALB/c mice injected intravenously with 4 X 10(7) ffp were substantially protected against a challenge infection with L. major promastigotes. The possible relationship between the suppressor T cells for DTH and prophylactic immunization against fatal cutaneous leishmanial infection in susceptible BALB/c mice is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benacerraf B., Germain R. N. A single major pathway of T-lymphocyte interactions in antigen-specific immune suppression. Scand J Immunol. 1981;13(1):1–10. doi: 10.1111/j.1365-3083.1981.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Bradley D. J. Regulation of Leishmania populations within the host. II. genetic control of acute susceptibility of mice to Leishmania donovani infection. Clin Exp Immunol. 1977 Oct;30(1):130–140. [PMC free article] [PubMed] [Google Scholar]
- Bradley D. J., Taylor B. A., Blackwell J., Evans E. P., Freeman J. Regulation of Leishmania populations within the host. III. Mapping of the locus controlling susceptibility to visceral leishmaniasis in the mouse. Clin Exp Immunol. 1979 Jul;37(1):7–14. [PMC free article] [PubMed] [Google Scholar]
- DeTolla L. J., Scott P. A., Farrell J. P. Single gene control of resistance to cutaneous leishmaniasis in mice. Immunogenetics. 1981;14(1-2):29–39. doi: 10.1007/BF00344297. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Gill H. K., Dhaliwal J. S., Sukumaran K. D., Liew F. Y. Effect of irradiation on the precursor, activated and memory suppressor T cells for delayed-type hypersensitivity to sheep erythrocytes in mice. Immunology. 1984 Dec;53(4):669–675. [PMC free article] [PubMed] [Google Scholar]
- Gorczynski R. M., MacRae S. Analysis of subpopulations of glass-adherent mouse skin cells controlling resistance/susceptibility to infection with Leishmania tropica, and correlation with the development of independent proliferative signals to Lyt-1+/Lyt-2+ T lymphocytes. Cell Immunol. 1982 Feb;67(1):74–89. doi: 10.1016/0008-8749(82)90200-3. [DOI] [PubMed] [Google Scholar]
- Handman E., Ceredig R., Mitchell G. F. Murine cutaneous leishmaniasis: disease patterns in intact and nude mice of various genotypes and examination of some differences between normal and infected macrophages. Aust J Exp Biol Med Sci. 1979 Feb;57(1):9–29. doi: 10.1038/icb.1979.2. [DOI] [PubMed] [Google Scholar]
- Howard J. G., Hale C., Chan-Liew W. L. Immunological regulation of experimental cutaneous leishmaniasis. 1. Immunogenetic aspects of susceptibility to Leishmania tropica in mice. Parasite Immunol. 1980 Winter;2(4):303–314. doi: 10.1111/j.1365-3024.1980.tb00061.x. [DOI] [PubMed] [Google Scholar]
- Howard J. G., Hale C., Liew F. Y. Immunological regulation of experimental cutaneous leishmaniasis. III. Nature and significance of specific suppression of cell-mediated immunity in mice highly susceptible to Leishmania tropica. J Exp Med. 1980 Sep 1;152(3):594–607. doi: 10.1084/jem.152.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. G., Hale C., Liew F. Y. Immunological regulation of experimental cutaneous leishmaniasis. IV. Prophylactic effect of sublethal irradiation as a result of abrogation of suppressor T cell generation in mice genetically susceptible to Leishmania tropica. J Exp Med. 1981 Mar 1;153(3):557–568. doi: 10.1084/jem.153.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. G., Liew F. Y., Hale C., Nicklin S. Prophylactic immunization against experimental leishmaniasis. II. Further characterization of the protective immunity against fatal Leishmania tropica infection induced by irradiated promastigotes. J Immunol. 1984 Jan;132(1):450–455. [PubMed] [Google Scholar]
- Kellina O. I. O razlichiiakh v chuvstvitel'nosti inbrednykh myshei raznykh linii k Leishmania tropica major. Med Parazitol (Mosk) 1973 May-Jun;42(3):279–285. [PubMed] [Google Scholar]
- Liew F. Y., Howard J. G., Hale C. Prophylactic immunization against experimental leishmaniasis. III. Protection against fatal Leishmania tropica infection induced by irradiated promastigotes involves Lyt-1+2- T cells that do not mediate cutaneous DTH. J Immunol. 1984 Jan;132(1):456–461. [PubMed] [Google Scholar]
- Liew F. Y. Regulation of delayed-type hypersensitivity. VI. Antigen-specific suppressor T cells and suppressor factor for delayed-type hypersensitivity to histocompatibility antigens. Transplantation. 1982 Jan;33(1):69–76. [PubMed] [Google Scholar]
- Olobo J. O., Handman E., Curtis J. M., Mitchell G. F. Antibodies to Leishmania tropica promastigotes during infection in mice of various genotypes. Aust J Exp Biol Med Sci. 1980 Dec;58(6):595–601. doi: 10.1038/icb.1980.61. [DOI] [PubMed] [Google Scholar]
- Preston P. M., Behbehani K., Dumonde D. C. Experimental cutaneous leishmaniasis: VI: anergy and allergy in the cellular immune response during non-healing infection in different strains of mice. J Clin Lab Immunol. 1978 Nov;1(3):207–219. [PubMed] [Google Scholar]
- Preston P. M., Dumonde D. C. Experimental cutaneous leishmaniasis. V. Protective immunity in subclinical and self-healing infection in the mouse. Clin Exp Immunol. 1976 Jan;23(1):126–138. [PMC free article] [PubMed] [Google Scholar]
- Shand F. L. Analysis of immunosuppression generated by the graft-versus-host reaction. I. A suppressor T-cell component studied in vivo. Immunology. 1975 Dec;29(6):953–965. [PMC free article] [PubMed] [Google Scholar]
- Tada T., Okumura K. The role of antigen-specific T cell factors in the immune response. Adv Immunol. 1979;28:1–87. doi: 10.1016/s0065-2776(08)60799-3. [DOI] [PubMed] [Google Scholar]
- Titus R. G., Lima G. C., Engers H. D., Louis J. A. Exacerbation of murine cutaneous leishmaniasis by adoptive transfer of parasite-specific helper T cell populations capable of mediating Leishmania major-specific delayed-type hypersensitivity. J Immunol. 1984 Sep;133(3):1594–1600. [PubMed] [Google Scholar]


